Benjamin I. Brown
Abstract
Introduction
Methods
Towards precision nutrition for migraine
Healthy food plans
Migraine trigger foods
Gluten-free diet
IgG-based elimination diet
Low-histamine diet
Weight-loss diet
Low-glycaemic-index/ -load diet
High-omega-3/low-omega-6 diet
Ketogenic diet
Riboflavin
Niacin
Vitamin B12
Homocysteine-lowering vitamins:folate, vitamin B6 and vitamin B12
Vitamin E
Vitamin D
Magnesium
Abstract
Chronic migraine headaches are estimated to affect between 1.4% and 2.2% of the population, and have a huge impact on wellbeing and quality of life. A diverse range of therapeutic nutritional options have been explored for migraine headaches, with varying degrees of benefit. Dietary interventions include generally healthy food plans, identification and avoidance of trigger foods, weight-loss diets, low-glycaemic-load diets, ketogenic diets, gluten-free diets, IgG-led elimination diets and a high-omega-3/low-omega-6 diet. Nutritional supplement interventions include riboflavin, niacin, homocysteine-lowering B vitamins, vitamin B12, vitamin E, vitamin D, magnesium, zinc, iron, omega-3 fatty acids, coenzyme Q10 (CoQ10), lipoic acid, soy phytoestrogens, ginger, turmeric, carnitine, 5-hydroxytryptophan (5-HTP), palmitoylethanolamide (PEA), and multi-ingredient formulas. The wide range of therapeutic options may make it challenging to approach nutritional management of migraine in a clinical setting, so a pragmatic model that helps personalise interventions from clinical signs and symptoms and reliable biomarkers would be useful, so-called ‘precision nutrition’. The aim of this narrative review is to explore the clinical evidence for nutritional medicine for migraines, including diet and nutrient-based interventions, from the perspective
of personalised or precision nutrition.
Cite as: Brown, B. (2022) Migraine headaches: opportunities for management with precision nutrition. Nutr. Med. J., 1 (3), 117-152.
Affiliation: B. Brown is with the Nutritional Medicine Institute, London, UK, and the British College of Nutrition and Health (BCNH), London, UK.
Corresponding author: Benjamin I. Brown (email ben@nmi.health).
Article history: Received 16 August 2022; Peer-reviewed and received in revised form 4 September 2022; Accepted 4 September 2022. Available online 30 September 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Migraine headaches are recurrent headaches characterised by painful attacks lasting 4−72 hours. Chronic migraines are estimated to affect between 1.4% and 2.2% of the population, and have a huge impact on wellbeing and quality of life.1,2 The frequencies of migraine attacks are typically high, with 31.3% experiencing three or more attacks per month, and attacks are often severe, with 53.7% reporting severe impairment or being bedbound during the attacks.3
The diagnosis of migraine is based on clinical criteria, in particular recurrent headache attacks of unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity, and association with nausea, vomiting, photophobia and phonophobia.4 Although synonymous with headache attacks, migraine is more appropriately considered a symptomatically diverse neurological disorder, of which headache is just one symptom.5 During the premonitory stage up to 3 days before headache, tiredness, mood changes, yawning, thirst, cravings, urinary frequency, light and sound sensitivity, and cranial autonomic symptoms may occur, while symptoms in the postdrome phase up to 48 hours post-headache include tiredness and difficulty concentrating.6
The development of chronic migraine is understood to be a gradual process, with predisposing risk factors interacting with genetic susceptibility and frequent headache pain to lower the threshold of migraine attacks and consequently increase the risk of chronic migraine.7,8 The pathophysiology of migraine varies between individuals, but evidence does suggest features that distinguish migraineurs from non-migraineurs in the interictal phase (between headaches), including neuronal hyperexcitability,9 systemic oxidative and nitrosative stress,10 systemic low-grade inflammation,11 and altered blood glucose metabolism.12 Neurobiological changes are evident in the premonitory stage and include alterations in nociceptive signalling, while the headache phase involves activation of the trigeminovascular system and, in migraine with aura, a cortical spreading depression-like event characterised by an electrophysiological wave of excitation followed by inhibition in cortical neurons.13 Better understanding of the complex pathophysiology and underlying pathophysiological mechanisms of migraine may improve treatment options.14
Over-the-counter and prescription medications for both acute relief and prevention are common treatments for migraine, but treatment response is often poor and there are important potential side-effects, thus pharmacological treatment options are limited and only result in reduction of symptoms in a portion of migraine sufferers.15,16
Management of migraine would benefit from an approach that extends beyond pharmacotherapy alone, and identifies exacerbating factors and relevant comorbid conditions, examines past and current medications and other therapies, and a management plan with preventive, acute and lifestyle components as well as self-monitoring with a headache diary.17 Personalised lifestyle medicine, including sleep hygiene, stress management, aerobic exercise and dietary modification as well as nutraceuticals, should be considered in a migraine management plan.18,19,20 The aim of this narrative review is to explore the clinical evidence for nutritional medicine for migraine, including diet and nutrient-based interventions, from the perspective of personalised or precision nutrition.
Methods
The author searched PubMed for publications describing human clinical interventions involving diet, nutrient-based supplements, plant extracts with common food use, and migraine. There was no limit on date of publication. Articles assessing the effects of diet, nutritional and nutraceutical interventions in patients with migraine were included in the final review. Phytomedicines including feverfew (Tanacetum parthenium) and butterbur (Petasites hybridus) were excluded from the review, except where they were used as components of nutrient-based interventions, and are reviewed in detail elsewhere.21 Citations in these articles were also reviewed and located when related to additionally relevant clinical trials. Some articles not indexed on PubMed were identified and included if they were from a peer-reviewed journal. The review is based on this search and the author’s collection of studies on the topic used in previous academic and continuing professional education lectures. Only studies published in English were included in the review.
Towards precision nutrition for migraine
A diverse range of therapeutic nutritional options have been explored for migraine headache, with varying degrees of benefit. Dietary interventions include generally healthy food plans, identification and avoidance of trigger foods, weight-loss diets, low-glycaemic-load diets, ketogenic diets, gluten-free diets, IgG-led elimination diets, and a high-omega-3/low-omega-6 diet. Nutritional supplement interventions include homocysteine-lowering B vitamins, niacin, vitamin B12, vitamin E, vitamin D, magnesium, zinc, iron, omega-3 fatty acids, riboflavin, coenzyme Q10 (CoQ10), lipoic acid, soy phytoestrogens, ginger, turmeric, carnitine, 5-hydroxytryptophan (5-HTP), palmitoylethanolamide (PEA), and multi-ingredient formulas. The wide range of therapeutic options may make it challenging to approach nutritional management of migraine in a clinical setting, so a pragmatic model that helps personalise interventions from clinical signs and symptoms and reliable biomarkers would be useful, so-called ‘precision nutrition’.22 Such a model may also be informative for future clinical research that attempts to explore precision nutrition approaches for migraine. The structure of this review is based on a preliminary and theoretical model that incorporates and organises nutritional interventions into specific clinical and biochemical indications; however, it should be emphasised that this model is hypothetical and requires further evaluation before being translated to practice (Figures 1 and 2).
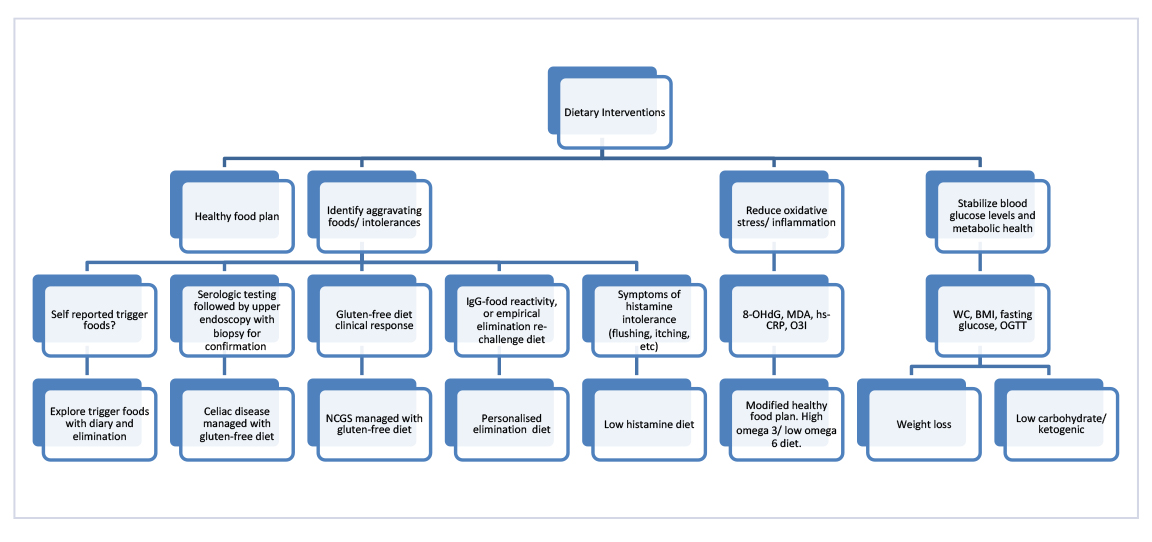
Figure 1: Dietary interventions for migraine.
Figure 1: Dietary interventions for migraine. Dietary interventions can be broadly divided into three major categories that may also be indications for their clinical use: adopting a generally healthy diet; identifying aggravating foods or intolerances; and modifying underlying pathophysiology or dysfunction. Clinical indications, such as dietary history, and biomarkers or results of investigations, such as the positive confirmation of coeliac disease, may help identify which dietary interventions are best suited to an individual. 8-OHdG, 8-hydroxy-2′-deoxyguanosine; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; NCGS, non-coeliac gluten sensitivity; O3I, Omega-3 Index (red blood cell omega-3 fatty acids); OGTT, oral glucose tolerance test; WC, waist circumference.
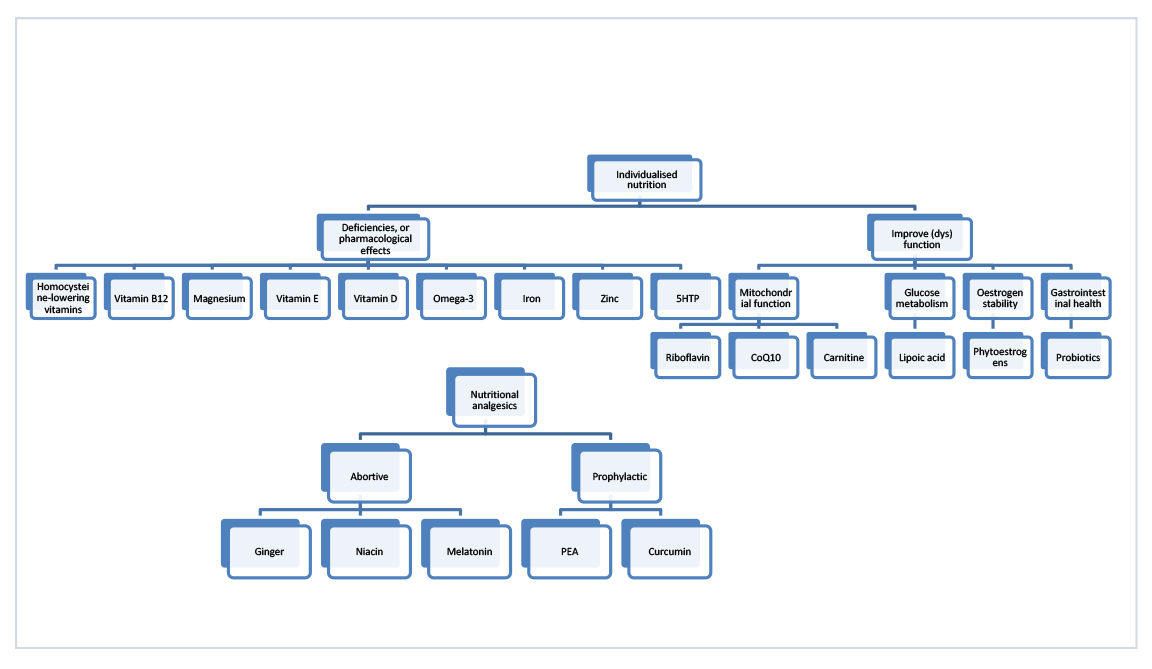
Figure 2: Individualised nutrition for migraine.
Figure 2: Individualised nutrition for migraine. A broad range of nutritional interventions is possible, so a pragmatic model that rationalises their indications and use may be helpful in clinical practice. Suboptimal intakes or deficiencies of vitamins, minerals and fatty acids can be assessed through dietary recall and/or laboratory testing, although both have limitations and may not accurately identify treatment responders, and nutrients may have pharmacological effects independent of deficiency or low dietary intake. Other nutrients have more specific indications like improving mitochondrial function, blood glucose metabolism or oestrogen stability; however, it should be emphasized that nutritional compounds have diverse mechanisms of action so this may be an oversimplification. 5-HTP, 5-hydroxytryptophan; CoQ10, coenzyme Q10; PEA, palmitoylethanolamide.
Healthy food plans
It has been speculated that healthful dietary changes could positively influence several aspects of migraine pathophysiology, including serotoninergic dysfunction, neuronal excitability, redox stress, brain mitochondrial function, neuroinflammation and platelet aggregation.23 Observational studies have suggested a relationship between food pattern and migraine. Higher diet quality, calculated with the Healthy Eating Index, was found to be significantly inversely associated with chronic migraine and headache attack frequency in women.24,25 Similarly, a higher Dietary Inflammatory Index score, and thus higher dietary inflammatory potential, has been found to be associated with migraine and headache frequency.26,27 Also a higher Dietary Diversity Score, a proxy indicator of nutritional adequacy, was inversely associated with migraine disability, pain severity and headache frequency in another study.28 In addition to food relationships, better hydration has also been associated with lower migraine severity.29
Some dietary intervention studies have assessed the impact of improving overall diet quality in migraineurs, and those that have been performed do suggest healthier eating could result in important clinical benefits for some people. A 90-day intervention (n = 52) based on healthy dietary guidelines for the Brazilian population, and personalised for body weight, improved dietary habits, dietary quality, and significantly reduced migraine severity and depressive symptoms, independent of changes in body weight.30
A retrospective study of the impact of a 3-month dietary intervention (n = 30; based on the Healthy Eating Plate created by Harvard School of Public Health) on migraine found a significant improvement in headache frequency, disability scales and use of abortive drugs per month (6.07 ± 7.83 versus 9 ± 8.2 at baseline).31 Patients statistically ate less sweets, white bread, and red or processed meat, and more whole cereals and legumes, fish, snacks (mainly with yogurt), and also water intake increased to 1.5 L a day. A second larger 12-week study (n = 97; also based on the Healthy Eating Plate) found that those who changed their eating behaviours had a statistically significant reduction in monthly migraine days (from 10 to less than 6) and a non-significant decrease in monthly painkiller intake (from approximately 12 to 8), with reductions in total carbohydrate, and red and processed meat consumption appearing to have the most pronounced benefit.32
Dietary changes characteristic of overall healthy eating patterns have individually demonstrated some benefit, including moderating fat intake and increasing plant foods. Moderating fat intake (< 20 g daily) for 12 weeks was found to reduce migraine frequency, intensity and duration, and medication intake.33 Reducing fat intake to < 20% of total calories, but not less than 45 g daily and mostly from olive oil, for 3 months significantly reduced the frequency and severity of headache attacks.34 A whole-food plant-based (vegan) diet was reported to result in complete remission of migraine after 3 months in one patient.35
Overall, there is preliminary but compelling evidence to suggest that nutrition education and counselling with subsequent improvements in dietary quality can have an important impact on migraine frequency and severity, and can reduce medication requirement.
Migraine trigger foods
A variety of foods and food ingredients can immediately (within hours) or chronically (within days or weeks) trigger migraine attacks in susceptible individuals, with anywhere from 12% to 60% of migraine sufferers reporting food as a trigger.36 Several have been identified, including alcohol (especially red wine and beer), chocolate, coffee, caffeine, dairy products (especially aged cheese and processed products such as ice-cream), processed meats, citrus fruits, tomatoes, onions, nitrates and nitrites, biogenic amines (such as histamine, tyramine and phenylethylamine), monosodium glutamate (MSG), aspartame, sucralose, and high or low sodium intake.37,38,39 Of these, caffeine withdrawal and MSG exposure have the strongest evidence as triggers because they are supported by positive provocation studies.40 The mechanisms by which foods and their components may contribute to migraine are +diverse, and appear to depend on individual susceptibility, dosage and timing of exposure.40
Studies examining the effects of avoiding trigger foods have produced mixed results. For example, in people with self-reported chocolate-triggered migraines, chocolate triggered migraine more frequently than placebo in one study,41 but not in others.42,43 Chocolate does contain caffeine, so caffeine withdrawal may have been a confounder. Similarly, when compared with placebo, aspartame administration was found to increase headache frequency in some,44,45 but not all studies.46,47 Clinically, however, identification and avoidance of trigger foods is an important component of nutritional therapy for migraine, and can be based on observation if the food trigger is clear or a food diary if less obvious. Alternatively, suspected triggers could be included in an elimination and re-challenge diet. Identification and elimination of trigger foods has been shown to be a useful clinical strategy. In one study, a group of migraineurs (n = 50) who suspected food triggers was split into two groups, and embarked on dietary restriction for 2 or 4 months. At 2 months, for both groups, monthly attack frequency, attack duration and attack severity were significantly decreased, and this benefit was maintained at 4 months only in the group who continued to avoid trigger foods.48 The same research group assessed this approach in elderly patients with migraine (n = 31) and found similar benefit, with significantly lower frequency of attack, attack duration, pain severity, and analgesic and triptan use at 2 months.49 Also, an intervention (n = 42) based on a low-fat, plant-based diet that incorporated elimination of common trigger foods found a significant reduction in headache intensity and pain; however, issues related to study design such as the use of a vegan diet, short duration of elimination and a non-personalised approach to trigger food elimination limit the generalisability of this particular study.50 Overall, this clinical evidence shows that identification of trigger foods with a food diary and subsequent avoidance may be a useful strategy for some people with migraine.
Gluten-free diet
Chronic and migraine headaches have been reported as a common presentation in coeliac disease, with a prevalence of up to 30%.51,52,53 Also, gastrointestinal symptoms are more frequent in coexisting migraine and coeliac disease when compared with coeliac disease alone.54 Importantly, gluten sensitivity may initially present with extra-intestinal symptoms in the absence of gastrointestinal symptoms or enteropathy, and has been associated with neurological disorders, including migraine.55,56
Gluten-free diets have been successful in reducing headaches and migraine in some studies. In one report of 10 people with episodic headache and gluten sensitivity, defined by the presence of antigliadin antibodies as well as HLA-DQ2 or HLA-DQ8 genotype, nine responded to a gluten-free diet.57 And in a group of people with coeliac disease (n = 90) who were treated with a gluten-free diet, three out of four people who initially had migraine attacks had an improvement in frequency, duration and intensity, while the remaining one became migraine free.58 A survey of people (n = 866) with headache and coeliac disease revealed that 24% reported headache as the main symptom that led to diagnosis of coeliac disease and, after initiation of a gluten-free diet, the frequency and intensity of migraine headaches significantly improved; symptoms relapsed on gluten exposure.59 In contrast, one investigation found that 28% of patients with coeliac disease (n = 72) had migraine despite a persistent gluten-free diet.60
Non-coeliac gluten sensitivity (NCGS) has been suggested to play a role in migraine, but evidence is currently limited. A significantly higher incidence of migraine has been reported in people with NCGS compared with controls.61 A preliminary study identified a subset of migraine patients with NCGS (n = 10) who, after commencing a gluten-free diet for 3 months, experienced a significant reduction in migraine disability.62 Additionally, a gluten-free diet has been shown to reduce symptoms of headache in people with suspected NCGS.63
Because migraine may be an extra-intestinal manifestation of coeliac disease, testing for coeliac disease if indicated may help identify an underlying contributory dietary factor in some people with migraine, which in some cases could improve headache symptoms after initiation of a strict gluten-free diet.64 If suspected, NCGS is determined with clinical response to a gluten-free diet for at least 6 weeks and subsequent blinded gluten challenge.65
IgG-based elimination diet
Diet restriction based on IgG antibodies may be a useful strategy for reducing the frequency of migraine attacks, with evidence from clinical studies generally reporting beneficial effects.66 The first evidence came from a proof-of-concept study in which migraine patients (n = 39) embarked on an IgG-based elimination diet with 30−40% reporting clinical benefit.67 In another study, 43 out of 65 patients with migraine refractory to traditional treatment had a complete remission of their migraine after 1−6 months of an IgG-based elimination diet.68 In the first randomised-controlled clinical trial, a 6-week IgG-based elimination diet (n = 30) found a significant reduction in the number of headache days and number of migraine attacks in the elimination diet period.69 However, in contrast, one study (n = 138) reported a significant reduction in migraine-like headaches with an IgG-based elimination diet at 4 weeks, but only a small non-significant reduction at 12 weeks of dietary therapy.70 This study lends only modest support for an IgG-based elimination diet, but may have been limited by differences in study design such as participants having self-reported migraine and thus possibly other forms of headache, lack of dietary support for participants compared with other trials, and inability to measure dietary adherence.
People with irritable bowel syndrome (IBS) are at higher risk for migraine,71 and gastrointestinal symptoms may respond to IgG-based elimination diets.72 In patients with a co-diagnosis of migraine and IBS (n = 21), a double-blind, randomised-controlled, crossover clinical trial reported significant reductions in symptoms of both disorders, including headache frequency, severity and duration, and gastrointestinal pain and bloating after a 6-week IgG-based elimination diet.73 In another study of a group of patients with migraine and IBS (n = 60), a 14-week IgG-based elimination diet with a probiotic was more effective than a control diet with a probiotic for reduction of migraine and gut symptoms.74 Overall, it appears that an IgG-based elimination diet may be useful in cases of migraine related to suspected food sensitivities.
Empirical oligoantigenic diets, also known as elimination re-challenge diets, were examined for migraine as early as the 1980s, but since then have received little attention.75 In a pioneering study involving migraine patients (n = 9) with suspected food triggers, Monro et al. mitigated symptomatic and immunological food reactivity with an antihistamine,76 and then went on to report that an elimination re-challenge diet, followed by an antigen desensitisation regime, was able to reduce symptoms in people with migraine (n = 11).77 Another research group reported that an elimination re-challenge diet resulted in recovery from migraine in 93% of a paediatric population (n = 88).78 The same research group reported benefit of the same dietary approach in children with migraine and epilepsy or attention-deficit hyperactivity disorder.79,80 In another study of a group of adults with migraine (n = 43) who were treated with an elimination re-challenge diet, 13 had a 66% or greater reduction in headache frequency, and six became headache free.81 Elimination re-challenge diets appear to be a useful clinical approach, but would benefit from renewed interest and research.
Low-histamine diet
Elevations in histamine due to an imbalance between detoxification and dietary exposure can cause histamine receptor-mediated complications and allergic-type symptoms, including headaches and migraine. Typically, histamine intolerance (HIT) presents with symptoms such as asthma-like symptoms, rhinitis, urticaria and gastrointestinal symptoms, including diarrhoea and abdominal pain, after ingestion of foods with a high histamine content.82 Histamine is found at a high concentration in aged foods (e.g. cheeses, alcoholic beverages, cured meats, fermented or spoiled foods), where it is produced by bacterial or yeast fermentation of the amino acid histidine to histamine. Other foods, such as citrus fruits, may have the capacity to enhance histamine release, even though they contain low levels of histamine themselves.83
Detoxification of dietary histamine normally occurs in intestinal epithelial cells via the enzyme diamine oxidase (DAO). If DAO fails to inactivate histamine, it is absorbed through the gut epithelium and enters systemic circulation where it may contribute to typical symptoms of HIT in some people.84 There have been associations between genetic variants that affect DAO activity and migraine headache, suggesting increased susceptibility to HIT in genetically predisposed individuals.85 Furthermore, blood DAO levels were found to be more frequently deficient (defined as levels below 80 HDU/ml) in migraine patients compared with controls (87% versus 44%).86
While there appear to be no studies of a low-histamine diet in migraine sufferers, one study found that pre-treatment with an oral anti-histamine prevented headaches after ingestion of a high-histamine food in people with HIT.87 A potential role for a low-histamine diet is also suggested by a clinical trial of DAO enzyme supplementation in patients with migraine (n = 100), which reported a significantly reduced duration of migraine attacks after 1 month of treatment.88 If clinical assessment suggests HIT, a low-histamine diet or therapeutic trial of DAO supplementation may be warranted.89
Weight-loss diet
Obesity can increase the risk for migraine as well as exacerbate migraine frequency and severity, a relationship that may be in part due to amplification of the inflammatory response in migraine.90 Nutritional and behavioural weight loss is useful for people with comorbid migraine and obesity as it can reduce migraine severity and impact.91 In one study (n = 135), a year-long weight-loss intervention that included diet, physical activity and cognitive behavioural training was found to significantly decrease body weight, headache frequency and intensity, use of acute medications, and disability within 6 months in obese adolescents with migraine. Furthermore, benefits persisted at least 12 months after treatment.92 While diet and lifestyle counselling and subsequent weight loss may be beneficial, it is also possible that diet and physical activity, independent of weight change, may mitigate migraine.
Low-glycaemic-index/-load diet
Poor blood glucose regulation, especially reactive hypoglycaemia and hyperinsulinaemia, has been associated with migraine attacks, with several early studies reporting beneficial effects of low-carbohydrate diets.93,94 In one such study, dietary therapy (a low-sugar diet and six meals daily) resulted in at least 75% reduction of frequency of attacks in all diabetic patients (100%), and most migraine sufferers (63%) who previously demonstrated reactive hypoglycaemia.95 More recently, a clinical study of a very-low-carbohydrate ketogenic diet and a case report of a low-carbohydrate diet reported important clinical benefits in migraine sufferers, further supporting the rationale for a low-carbohydrate diet in migraine associated with dysglycaemia.96,97 Also, a low-glycaemic-index diet significantly decreased monthly attack frequency and migraine severity in patients (n = 147) after 3 months.98 Overall, patients with migraine, and especially those with concomitant dysglycaemia, could benefit from a low-glycaemic-index/-load diet.
High-omega-3/low-omega-6 diet
An increased ratio of dietary omega-6 to omega-3 fatty acids in industrialised diets has been proposed to contribute to inflammatory disorders, including migraine.99 In support of this, a high plasma omega-6 to omega-3 ratio was more strongly positively correlated with elevations in inflammatory mediators than levels of each fatty acid alone.100 In a clinical study (n = 56), a combination of increasing dietary omega-3 with concurrent reduction in omega-6 fatty acids produced statistically clinically relevant improvements in headache hours per day, severe headache days, headache-related quality of life, and reduction in use of migraine-specific medications.101 There was also a reduction in psychological distress and an improvement in health-related quality of life and function.102 A subsequent study was able to demonstrate that increasing dietary omega-3 with concurrent reduction in omega-6 fatty acids is more effective than increasing dietary omega-3 alone, suggesting that the dietary omega-6 to omega-3 fatty acid ratio is indeed important.103 The suitability of a dietary intervention that improves omega-3 to omega-6 fatty acid ratios could be based on dietary assessment or laboratory values of fatty acid status.
Ketogenic diet
Ketogenic diets have a long history of investigation in neurological diseases and have a variety of mechanisms that may explain their clinical effects, including improvement of brain energy metabolism, mitochondrial function, redox balance, reduction of neuroinflammation, pro-excitatory and inhibitory neurotransmitters, and modulation of the gut microbiome.104,105
The use of ketogenic diets for migraine dates back to 1928 when an investigative clinical trial found 9 out of 23 patients (39%) with migraine responded well to a ketogenic diet.106 Then in 1930 a study reported a reduction in headache frequency of at least 50% in 75% of patients, and total remission of headache in 50% of patients with migraine.107
More recently the use of ketogenic diets for migraine has been once again investigated, in part due to the publication of a few case reports describing beneficial effects that led to larger scale investigations.108,109 In a prospective observational study (n = 52), a ketogenic diet intervention had a 90% treatment response rate and reduction in medication use over a 1-month period while, in contrast, a standard low-calorie diet had no effect.110 A proof-of-concept study (n = 45) found a significant reduction in attack frequency, number of days with headaches, and medication use intake over 1 month.111 A randomised double-blind, crossover trial (n = 35) comparing the effects of a very-low-calorie ketogenic diet and a very-low-calorie non-ketogenic diet found the ketogenic diet had a 50% better response rate for days with migraine (74% versus 8%), and was superior for reducing the number of days with migraine (−3.73 days) and migraine attacks (−3.02).112
In a 12-week randomised-controlled crossover trial, a ketogenic diet lowered migraine duration, although the effect was not statistically significant.113 However, there was a high dropout rate with only 11 out of 26 people completing the study, and all participants reported fatigue as a side-effect. In another study (n = 38), patients with drug refractory chronic migraine and medication overuse headache who were treated with a ketogenic diet for 3 months had a decrease in days with symptoms (from 30 days to 7.5 days), migraine duration (from 24 hours to 5.5 hours), migraine pain (55% response rate), and medication use (from 30 doses a month to 6 doses a month).114 In two trials, patients with refractory migraine (total n = 48) treated with a ketogenic diet had a significant reduction in the frequency of migraine attacks, intensity of headache and medication use when compared with a control diet with a similar reduction of carbohydrate.115
Exogenous beta-hydroxybutyrates (ketones) have been proposed as an alternative to ketogenic diet therapy, but a clinical trial assessing supplementation with beta-hydroxybutyrate (7.4 g daily for 12 weeks) found no benefit for migraine frequency or intensity.116 In contrast, medium-chain triglycerides (MCTs), which are sometimes used as a component of ketogenic diet therapy, were shown to have potential in one study. Treatment with MCTs (13 g total fat per serving from coconut oil) for 30 days with no other dietary changes (n = 14) reduced the number of migraine episodes (−39%) and migraine duration (−61%).117
There is promising evidence for ketogenic diets for migraine, but clinical trials to date suggest they need to be strictly ketogenic and not very-low-calorie or low-carbohydrate diets as these appear not to produce the same therapeutic effects. Furthermore, ketogenic diets for migraine may differ from ketogenic diets for other neurological disorders or those used for weight loss due to unique dietary triggers, and different ketogenic ratio (ratio between fats and carbohydrates + proteins) requirements. Di Lorenzo et al. have published clinical guidelines and recommendations.118
Riboflavin
Riboflavin has been shown to reduce migraine frequency. Several mechanisms may explain the therapeutic effect of riboflavin, including reduction of oxidative stress, improvement in mitochondrial function, amelioration of neuroinflammation and modulation of glutamate excitotoxicity.119 A meta-analysis of 11 clinical trials in adolescents and adults concluded that riboflavin is well tolerated and that most clinical trials have shown modest reductions in migraine headache frequency after 2−3 months. However, benefits in children and adolescents were inconclusive.120
The optimal dose of riboflavin for migraine prevention is uncertain. Clinical studies in adolescents have typically used doses ranging from 50 mg to 400 mg daily,121,122,123,124,125 while adult studies used doses ranging from 100 mg to 400 mg daily.126,127,128,129 Lower doses may be as effective as higher doses but are not as well studied. In adolescents, as little as 10 mg of riboflavin daily was found to be effective for migraine prevention in one study,130 and in adults a dose of riboflavin 25 mg daily was as effective as a combination nutritional formula providing 400 mg of riboflavin with magnesium and feverfew.131 The maximal absorption of riboflavin after doses of up to 60 mg is 27 mg,132 so it is plausible that there is no advantage to higher doses but this needs to be tested in dose comparison studies.
It is unlikely that overt riboflavin deficiency explains the benefit of supplementation for migraine.133 However, it is possible that people prone to migraine have a higher requirement for riboflavin, with one study suggesting that riboflavin may be more effective in patients with certain mitochondrial DNA genetic variations, specifically those who did not have mitochondrial haplogroup H.134 This observation is consistent with the notion that individuals have wide variations in micronutrient requirements that are genetically determined.135
Niacin
Niacin has been reported to prevent migraine and to be used acutely to avert migraine attacks, although research is limited. Several reports of intravenous (IV) and oral niacin have described important clinical benefits for migraine and tension-type headaches, including for aborting acute attacks.136
It has been proposed that niacin may be a useful acute treatment due to its vasodilatory effect, which may counter the cranial vasoconstriction associated with the development of migraine aura and pain.137 In case reports, 500 mg of oral niacin taken at the onset of acute symptoms prevented migraine.138 And in another case report, 375 mg of sustained-release niacin 1−2 times daily for 3 months prevented migraine.139 A subsequent systematic review identified 11 clinical reports describing positive effects of IV or oral niacin for headache and migraine headache prevention and acute treatment.140
Vitamin B12
Vitamin B12 status has been found to be significantly associated with migraine, and correction of deficiency could resolve headaches in some people. Vitamin B12 deficiency has been associated with elevated serum tumour necrosis factor-alpha (TNF-α) and treatment with vitamin B12 lowers TNF-α, suggesting inflammation may underlie the relationship.141 Vitamin B12 also possesses antioxidant properties, and subclinical deficiency has been associated with oxidant stress.142
In a study of patients with migraine (n = 140), those with serum vitamin B12 in the highest quartile had an 80% decrease in the odds of having migraine, while those in the highest quartile of methylmalonic acid (a biomarker of B12 deficiency) had a more than five times increased risk of having migraine.143 Relevant to migraine patients using analgesics, aspirin use may contribute to vitamin B12 deficiency by damaging the gastric mucosa and interfering with vitamin B12 absorption.144
Several studies suggest that vitamin B12 supplementation could be useful for preventing migraine, but are limited by small sample sizes and administration of vitamin B12 with other vitamins in most studies.145 In one study (n = 20), treatment with hydroxocobalamin (1000 mcg daily, intranasally) for 3 months was reported to reduce migraine attack frequency, duration of attacks, total number of migraine days and the number of medication doses for acute treatment used in treatment responders.146 No relationship between cobalamin serum concentrations and efficacy was observed, suggesting a pharmacological effect independent of B12 deficiency. Identification of vitamin B12 deficiency in children and adolescents with tension-type headaches and subsequent correction with 3 months of supplementation was found to completely resolve headaches, anaemia and concomitant anxiety.147
Homocysteine-lowering vitamins: folate, vitamin B6 and vitamin B12
Folate, vitamin B6 and vitamin B12 supplementation for migraine is supported by several clinical studies, and could be personalised based on measurement of homocysteine, while the presence of certain 5,10-methylenetetrahydrofolate reductase (MTHFR) polymorphisms may result in a better clinical response to supplementation in some people.148
Elevated serum homocysteine is a frequent finding in migraine sufferers, especially migraine with aura.149 Studies examining associations between MTHFR C677T and A1298C polymorphisms and migraine have been mixed, but a meta-analysis of 26 studies suggested that they may be a risk factor for migraine in Caucasians, but not Asian populations.150 Folate, vitamin B6 and vitamin B12 (as well as riboflavin and choline) lower elevated homocysteine, and individuals with MTHFR polymorphisms may be uniquely susceptible to adverse effects related to low dietary intake of these vitamins.
In a review of five studies assessing supplementation with folate, vitamin B6 and/or vitamin B12, most studies reported good outcomes for migraine prevention.151 Genotype may modify treatment response. In an open-label study (n = 16), children with migraine, hyperhomocysteinaemia and MTHFR polymorphisms responded well to folic acid (5 mg once daily) for 6 months.152 In a subsequent clinical trial (n = 52), people with migraine and the MTHFR C677T genotype who were carriers of the C allele experienced a greater treatment response compared with TT genotypes in a 6-month clinical trial of folic acid, vitamin B6 and vitamin B12 supplementation (2 mg of folic acid, 25 mg of vitamin B6 and 400 mcg of vitamin B12).153 Similarly, women who were C allele carriers of the MTHFR C677T variant showed a higher reduction in homocysteine levels and severity of pain in migraine in response to the same dose of folic acid, vitamin B6 and vitamin B12 supplementation in another study (n = 206).154 Related to diet, a higher dietary intake of folate has been associated with reduced migraine frequency, particularly in individuals with the CC genotype for the MTHFR C677T.155
Vitamin E
Vitamin E has antioxidant activity in the central nervous system that may be relevant to the pathophysiological role of redox stress in migraine.156 A double-blinded trial in women with menstrual migraine (n = 72) found that vitamin E supplementation at a dose of 400 IU daily for 5 days, 2 days before to 3 days after menstruation, for two cycles significantly reduced pain severity, photophobia, phonophobia, nausea and functional disability when compared with placebo.157 In a clinical trial (n = 35), a vitamin E-containing antioxidant formula (providing N-acetylcysteine 1200 mg, vitamin C 1000 mg and vitamin E 500 IU per day) reduced the number of headaches per month (by 3 per month), migraine duration, headache pain scores, and acute headache medication use after 3 months of treatment.158
Vitamin D
Vitamin D deficiency or insufficiency has a higher prevalence in migraine sufferers compared with controls, and vitamin D supplementation might reduce the frequency of attacks. It has been estimated that vitamin D deficiency or insufficiency is present in 45−100% of people with migraine, and serum vitamin D level has been negatively correlated with frequency of headaches.159 In people with migraine, vitamin D supplementation has been shown to exert a number of biological effects relevant to migraine pathophysiology, including improved Th17/Treg related cytokines balance,160 lowering calcitonin gene-related peptide (CGRP),161 and reducing nitric oxide synthase and interleukin (IL)-6.162
Several clinical studies have assessed the effect of vitamin D in people with headache or migraine, and generally showed a decrease in headache frequency after vitamin D supplementation.163 For example, children with migraine (n = 53) who were given vitamin D (400 IU, 800 IU or 5000 IU daily if not deficient, mildly deficient, or very deficient, respectively) plus amitriptyline for 6 months had a significantly lower number of migraine attacks when compared with amitriptyline alone.164 Adults (n = 165) with migraine who received vitamin D (4000 IU daily) for 24 weeks had significantly raised serum 25(OH)D levels and reduced frequency of migraine attacks.165 Vitamin D may also improve medication treatment response, as noted in a report describing a positive interaction with pregabalin.166
Magnesium
Magnesium deficiency adversely affects neurological function and could contribute to the development of migraine.167 Magnesium’s blockade of the glutamatergic N-methyl-D-aspartate receptor, a receptor known to be a contributor to pain transmission, and its role in mitochondrial functioning are some mechanisms considered particularly relevant.168
Low serum, saliva and brain levels of magnesium are present in people with migraine, are exaggerated during an acute migraine attack, and significantly predict migraine intensity.169,170,171 In a population study, lower dietary intake of magnesium increased risk of migraine, while attainment of the Recommended Dietary Allowance through food and supplements was associated with reduced risk.172
A review of five clinical trials found that magnesium, when used as a prophylactic treatment against migraine, resulted in a favourable reduction in migraine attacks ranging between 22% and 43%.173 Evidence of efficacy was more compelling for a dose of > 600 mg daily and for magnesium as magnesium citrate when compared with other types of magnesium.
IV magnesium sulphate has been studied as a treatment for migraine in emergency department settings and is effective, comparable to other IV medications (metoclopramide, prochlorperazine, caffeine citrate), well tolerated and has a good safety profile.174,175,176,177
Iron
Iron-deficiency anaemia could contribute to migraine, and there is some evidence to suggest that treatment of iron-deficiency anaemia can greatly reduce the rate of migraine attacks suggesting it may be an underlying cause of migraine in some people.
Headache or migraine is not considered a typical symptom of iron-deficiency anaemia but may be overlooked. In a group of patients (n = 127) with iron-deficiency anaemia, 79.5% reported headaches and 36.2% met the criteria for migraine.178 A case−control study found an association between iron-deficiency anaemia and migraine, especially in women and girls.179 Iron-deficiency anaemia has also been significantly associated with menstrual migraine.180,181
Treatment of children (n = 98) with iron deficiency and migraine with iron (4 mg per kg per day of ferrous sulphate) for 3 months significantly reduced migraine frequency, severity, duration and disability.182 Iron supplementation has also been reported to reduce monthly frequency and severity of migraine headache in adults (n = 183) with migraine and iron deficiency.183 Dietary iron intake has been inversely associated with severe headache or migraine in women aged 20−50 years, suggesting optimising iron intake via diet may also be important for migraine prophylaxis.184
Zinc
Zinc serum levels have been observed as lower in people with migraine, 185,186 and this may have clinical relevance as zinc is essential for a wide range of neurological functions relevant to migraine.187 Clinical studies suggest zinc may be a useful therapy for migraine. A randomised clinical trial (n = 80) of zinc sulphate (50 mg zinc daily) for 8 weeks resulted in a reduction in headache severity and migraine attack frequency, although this was not statistically significant.188 A second study, also a randomised clinical trial (n = 60), assessed zinc gluconate (15 mg of zinc daily) over 12 weeks, and reported significantly reduced frequency and severity of migraine attacks.189
Coenzyme Q10 (CoQ10)
CoQ10 deficiency, as measured by serum CoQ10, is common in patients with migraine and can be used to personalise treatment.190 It is important to note that low serum CoQ10 is a biomarker of mitochondrial dysfunction, and that CoQ10 supplementation can improve mitochondrial function.191 CoQ10 also has well-documented anti-inflammatory effects, and has been shown to reduce the inflammatory markers CGRP and TNF-α in people with migraine.192
In a meta-analysis of six clinical studies, CoQ10 reduced the duration and frequency of migraine attacks.193 A dose−response analysis of CoQ10 in migraine suggested a non-linear association with maximal efficacy at 300 mg daily regardless of variation in bioavailability of different formulations.194 CoQ10 may be synergistic with curcumin, as one study (n = 100) found that a combination of curcumin (80 mg) plus CoQ10 (300 mg) was significantly more effective than either therapy alone for migraine prevention.195
Alpha-lipoic acid
Alpha-lipoic acid (ALA) has potential for the management of migraine, in part because it improves insulin sensitivity. Disordered glucose and insulin metabolism is a key feature of migraine, although it is not clear if alterations in glucose metabolism are risk factors for or consequences of migraine.196 Patients with migraine and insulin resistance who received ALA (400 mg twice daily) for 6 months experienced a reduction in the number of migraine attacks and days of treatment, although there was no improvement in insulin sensitivity in this study.197
ALA has antioxidant and anti-inflammatory effects, independent of effects on insulin metabolism, that may play a role in its therapeutic effect. In women with episodic migraine (n = 92), ALA (300 mg twice daily) for 3 months significantly decreased serum levels of malondialdehyde and C-reactive protein (CRP), and clinical symptoms of depression, anxiety and stress when compared with placebo.198
Improvements in mitochondrial and endothelial function have also been observed. In women with episodic migraines (n = 92), ALA (300 mg twice daily) for 12 weeks significantly reduced migraine severity and frequency, and reduced serum lactate and vascular cell adhesion molecule-1, suggesting improvement in mitochondrial and endothelial function, respectively.199
Other reports also suggest a therapeutic role for ALA. An open-label study (n = 54) reported a beneficial effect of ALA (600 mg daily) for migraine prophylaxis after just 1 month.200 When ALA (300 mg daily) was added to topiramate therapy, treatment was more effective and better tolerated than topiramate alone.201
Carnitine
Carnitine and acetyl-L-carnitine (ALC) have well-established neurological effects relevant to migraine, including mitochondrial enhancing, antioxidant and analgesic activity, but whether these translate to clinical value is less understood.202 The few studies assessing carnitine as a monotherapy have produced mixed results. A 12-week study (n = 72) of ALC (3 g daily) found no benefit for migraine prophylaxis in adults.203 In contrast, paediatric patients (n = 56) with episodic migraine treated with L-carnitine (50 mg per kg per day) experienced a reduction in migraine frequency, severity and duration that was comparable to the study arm receiving propranolol.204 Carnitine and ALC have been used as components of multi-ingredient nutritional formulations for migraine, and studies in which they have been administered with other nutrients are discussed below. Carnitine palmitoyltransferase II (CPT2) or carnitine deficiency should be considered in treatment refractory migraine. Case reports have described cases of migraine discovered to be related to CPT2 or carnitine deficiency, and successfully managed with carnitine in adolescents205,206 and young adults.207 In these cases, patients had chronic and often daily headache, additional diverse symptoms (e.g. chronic constipation, abdominal pain and constant extreme fatigue) and were refractory to multiple medications.
5-Hydroxytryptophan (5-HTP)
5-HTP has been investigated for migraine based on its role as a precursor to serotonin and the potential contribution of alterations in serotonin in the pathophysiology of migraine.208 Other potential mechanisms of 5-HTP in migraine include analgesic effects mediated by elevations in plasma beta-endorphins,209 and reaction in cortical spreading by serotonin−oestrogen interactions.210 However, studies of 5-HTP are few and were mostly conducted over 30 years ago with little interest in 5-HTP for migraine since this time, which coincides with the approval of the first selective serotonin reuptake inhibitor (SSRI) drugs.211 In a clinical study (n = 40), 5-HTP (300 mg daily for 40 days) was reported to reduce migraine frequency and severity compared with placebo.212 In patients with headache (n = 31), including migraine and other types of chronic headache, 5-HTP (400 mg daily) for 2 months showed that 48% of participants had a greater than 50% average reduction in headache symptoms.213 A study in patients with migraine (n = 124) assessed 5-HTP (600 mg daily) over 6 months with a significant improvement in 71% of patients, particularly for intensity and duration of attacks.214 In contrast, a 12-week study in a paediatric cohort with migraine found no benefit of 5-HTP (5 mg per kg of body weight daily) when compared with placebo.215 However, it should be noted that both 5-HTP and placebo significantly reduced migraine frequency and severity.
Clinical studies suggest that 5-HTP may be a useful therapy for migraine. It should also be noted that 5-HTP has been reported to benefit other types of headaches, including paediatric headaches,216 fibromyalgia-related migraine,217 chronic primary headaches218 and chronic tension-type headaches.219
Omega-3 fatty acids
Low dietary intake of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) increases the risk for headaches220 and migraine.221 An adequate dietary supply of EPA and DHA is critical for neurological health, and low levels, as measured by the percentage of EPA/DHA in erythrocytes [the Omega-3 Index (O3I)], have been associated with impaired brain function, atrophy of key brain regions and diverse neurological diseases.222
A systematic review and meta-analysis of 10 randomised-controlled trials of EPA/DHA in migraine found a significant reduction of approximately 3.44 hours in the duration of migraine, but no effect on frequency or severity.223 However, it was noted that there were several limitations that may have confounded results, including: lack of adequate placebo (olive oil was sometimes used); not controlling for omega-3:6 ratio (which may influence EPA/DHA efficacy); a masking effect by migraine medications; and lack of personalisation (e.g. not testing baseline omega-3 status or response). Subsequent to the publication of this meta-analysis, a clinical trial was published in which 66% of patients with chronic migraine who received omega-3 (1500 mg EPA/DHA daily) for 60 days in conjunction with amitriptyline had an 80.0% reduction in the number of headache days per month, compared with 33% with amitriptyline alone.224
In general, the evidence is suggestive for the benefits of EPA/DHA in migraine, but until better quality clinical trials are available that account for the limitations mentioned above the clinical benefit remains unclear. In practice, an exploratory therapeutic trial of 1000−2000 mg EPA/DHA daily for at least 8 weeks could be considered, and personalised based on testing of the O3I.225
Palmitoylethanolamide (PEA)
PEA is an endocannabinoid-like bioactive lipid mediator used as a nutritional supplement primarily for its neurological, anti-inflammatory and analgesic effects.226 Preclinical evidence suggests that modulation of the endocannabinoid system by PEA could relieve migraine pain.227 For pain management, case studies, clinical trials and meta-analyses of PEA have demonstrated good evidence of efficacy and excellent tolerability at typical doses of 600−1200 mg daily.228
In patients with migraine with aura (n = 20), PEA (1200 mg daily) for 90 days resulted in a statistically significant and time-dependent reduction in pain, as a composite of severity and frequency, at the 60- and 90-day time points.229 In addition, PEA reduced non-steroidal anti-inflammatory drug (NSAID) use compared with controls. An open-label study in patients with episodic migraine (n = 25) assessed a PEA-containing nutraceutical (Calmux®, Bioksan, Spain; providing PEA 800 mg, Scutellaria baicalensis 800 mg, Boswellia serrata extract 500 mg and Harpagophytum procumbens root extract 400 mg per day) over 3 months and reported a significant reduction in pain intensity.230 A paediatric cohort with migraine without aura (n = 70) who received PEA (600 mg daily) for 3 months experienced a significant reduction in pain intensity and attack frequency as well as a reduction in medication requirement.231
Melatonin
Melatonin, although a hormone, is available as a non-prescription over-the-counter dietary supplement in the USA, Canada and some EU countries. As a dietary supplement, melatonin is considered very well tolerated and safe at typical doses of 1−20 mg daily, and even safe when used at very high doses of up to 1000 mg daily in the short term.232 Relevant to migraine, serum melatonin may be lower in a sub-group of patients,233 and alterations in melatonin could play an important role in migraine pathophysiology via modulation of several neural pathways involved in the generation of migraine attacks.234 In addition, melatonin supplementation can exert analgesic effects independent of endogenous melatonin status.235 It is also possible that by improving sleep, melatonin may prevent migraine attacks,236 and help resolve acute attacks by encouraging sleep, at least in children.237
Studies suggest melatonin may be useful for migraine prevention, and for acute management of migraine headache attacks. In a systematic review of seven studies, melatonin was generally found to be effective for migraine prevention with comparable efficacy to other preventive medications. In adults, doses of 3 mg daily appeared to be more effective than 2 mg doses, and a treatment duration of 3 months was more likely to be related to a beneficial outcome.238
A novel study explored the use of melatonin for acute treatment of migraine attacks.239 Children and adolescents (n = 84) aged 4−17 years with episodic migraine were prescribed ‘high-dose’ or ‘low-dose’ melatonin (< 40 kg: 4 mg versus 1 mg; ≥ 40 kg: 8 mg versus 2 mg) as an edible milk-chocolate-based melatonin formulation (Good Day Chocolate, USA). Acute treatment of a migraine attack with melatonin reduced pain intensity and reduced migraine duration with a 2-hour pain-relief rate (defined as mild or no headache) of 94% and 80% in the high- and low-dose groups, respectively. Napping occurred in 67% and 47% in the high- and low-dose groups, respectively. Both higher dose and napping after treatment predicted greater benefit.
Ginger
Ginger (Zingiber officinale) has well-established clinical use as an anti-inflammatory and analgesic in addition to nausea, a common feature of migraine.240 A meta-analysis of three randomised-controlled studies (n = 227) in which ginger monotherapy (two studies) or feverfew (T. parthenium; one study) had been explored as an intervention for migraine prevention and acute treatment found ginger effective for reducing migraine pain, nausea and vomiting.241 The studies attributed to positive effects were trials in which ginger was used as an acute abortive treatment while, in contrast, ginger used as a daily prophylactic over 1 month did not perform better than placebo.242 The first study assessing ginger (with feverfew) as an abortive treatment was in fact a homeopathic remedy, and not a herbal product.243 In the second, patients treated with ginger (a single dose of 400 mg of extract providing 5% gingerols) in addition to IV ketoprofen showed significantly better clinical response after 1 hour, 1.5 hours and 2 hours compared with medication alone.244 Also, ginger reduced pain and improved functional status. Ginger appears to have promise as an acute abortive treatment, but this requires validation with further research. Clinically, a therapeutic trial of ginger at the first sign of headache could be considered.
Curcumin
Curcumin, an anti-inflammatory polyphenol derived from turmeric (Curcuma longa) rhizome, has been explored as a possible therapeutic intervention in patients with migraine, alone and in combination with other nutrients. In a clinical trial of nano-curcumin alone, patients with episodic migraine (n = 44) were randomised to nano-curcumin (80 mg daily) or placebo for 2 months.245 Nano-curcumin was reported to significantly reduce the frequency, severity and duration of headaches. The same research group have explored mechanisms of action in study participants, demonstrating modulation of the Th2/T regulatory cell axis,246 and reduction in gene expression and plasma levels of IL-17.247 Another research group studied a different curcumin extract (n = 44) for a period of 8 weeks at a dose of 500 mg twice daily (providing 95% curcuminoids), administered after lunch and dinner to improve absorption.248 In this study, participants (n = 44) receiving curcumin had a significant reduction in headache severity and duration, with a marginal reduction in frequency. There was also a significant reduction in CGRP and IL-6.
A series of studies have examined the clinical effect of a nano-curcumin and omega-3 fatty acid formulation. A clinical trial (n = 74) comparing nano-curcumin (80 mg daily), omega-3 fatty acids (1200 mg of EPA and 600 mg of DHA daily), a combination of them, or placebo found a significant reduction in migraine attack frequency in the nano-curcumin and omega-3 fatty acids combination, while curcumin or omega-3 fatty acids alone were not effective.249 In addition, they demonstrated a significant downregulation of TNF-α gene expression with the combination only. The same research group have also reported downregulation of IL-6 gene expression, a decrease in the serum concentration of high-sensitivity (hs)-CRP,250 reduction of serum intercellular adhesion molecule 1 (ICAM-1)251 and serum IL-1β,252 with the effect generally more pronounced for the combination.
A combination of nano-curcumin and CoQ10 has also been investigated. Patients with episodic migraine (n = 91) received either a combination of nano-curcumin (80 mg) plus CoQ10 (300 mg), nano-curcumin, CoQ10, or placebo for 8 weeks. The curcumin and CoQ10 combination was more effective than all the other interventions for reducing migraine attack frequency, severity and duration, suggesting a synergistic or additive effect.195
Overall, it appears curcumin alone or in combination with omega-3 fatty acids of CoQ10 may be useful for migraine, and both more commonly available 95% curcuminoid extracts and nano-formulations (e.g. liposomal) may be effective albeit at significantly different doses of 1000 mg (total extract) or 80 mg (curcumin) daily, respectively.253
Phytoestrogens
Based on the oestrogen withdrawal hypothesis of migraine aetiology, phytoestrogens have been explored as a therapeutic for migraines with some evidence of potential benefit. The oestrogen withdrawal hypothesis is based on the observation that migraine is more prevalent in women and tends to be related to fluctuations in oestrogen during menarche, menstruation, pregnancy and menopause, with migraine attacks being twice as common in the perimenstrual period when oestrogen levels decline.254 Oestrogen directly influences several pathways involved in migraine in the central nervous system.255 A higher rate of oestrogen decline in the late luteal phase and 2 days post-peak may represent a neuroendocrine vulnerability pattern in migraineurs versus controls.256 Stabilising oestrogen with hormone replacement therapy can help mitigate migraine.257 A possible alternative to hormone replacement therapy for the management of migraine is phytoestrogens; non-steroidal phytonutrients that selectively modulate oestrogen receptors.258
Three clinical trials have assessed the effect of phytoestrogens in menstrual migraine, including soy isoflavones with other hormone-modulating herbs,259 soy isoflavones alone,260 and resveratrol (Table 1).261
Table 1: Clinical trials assessing phytoestrogens for migraine prophylaxis
These studies suggest that soy isoflavone extract with additional herbs or the soy isoflavones genistein and daidzein alone may be useful for menstrual migraine, while resveratrol has no benefit. The reason for this difference in efficacy may be due to differences in ability to selectively modulate oestrogen receptors between soy isoflavones and resveratrol. Soy isoflavones are oestrogen receptor β-specific, while resveratrol modulates both α and β oestrogen receptors.262 Therefore, if trialling phytoestrogens, soy isoflavones at a dose of > 76 mg daily for at least 3 months should be considered.
Probiotics
Digestive disorders have frequently been associated with migraine headaches, and may play a contributory or causal role through a variety of mechanisms, including alterations in the enteric immune and nervous systems by intestinal bacteria and gut-derived inflammatory and vasoactive mediators entering circulation.262 In one study, migraine sufferers were found to have significantly higher levels of nitrate, nitrite and nitric oxide reductase bacterial genes in samples collected from the oral cavity, and slight but significant differences in faecal samples as well.263 Modification of gastrointestinal health and ecology could be a treatment target, with a number of clinical examples that support the importance of identifying and managing gastrointestinal dysfunction in migraine sufferers. Eradication of Helicobacter pylori,264,265,266 improvement of IBS with an IgG-based elimination diet, and treatment of coeliac disease with a gluten-free diet have resulted in improvement in migraine. In fact, many nutritional interventions discussed above describe dietary changes and nutritional supplements (e.g. vitamin D and omega-3 fatty acids) that may improve gastrointestinal health and ecology.267 Interestingly, an uncontrolled study suggested possible benefit in migraine sufferers who were managed with a nutritional plan focused on improving gastrointestinal assimilation and elimination using a combination of probiotics, nutrients and herbs.268
Probiotics have been investigated in patients with migraine. A 12-week pilot study (n = 27) of a multi-strain probiotic (Ecologic Barrier, Winclove Probiotics, Netherlands) at a dose of 2.5 billion CFU daily reported a significant reduction in migraine frequency and intensity; however, this study lacked a control group.269 In a subsequent randomised placebo-controlled trial (n = 63) of the same probiotic at a dose of 5 billion CFU for 12 weeks, the intervention did not reduce migraine, failing to support the previous open-label study.270 In addition, there was no impact of the probiotic on intestinal permeability or inflammation compared with placebo. In contrast, a different probiotic was associated with clinical benefit. A 10-week randomised placebo-controlled trial (n = 79) assessed a multi-strain probiotic (Bio-Kult, ADM Protexin Limited, UK) at a dose of 4 billion CFU daily.271 Compared with placebo, the probiotic significantly reduced migraine frequency, severity, duration and abortive drug use.
It is plausible that improvements in gastrointestinal health such as those associated with clinical management of H. pylori, IBS or coeliac disease may coincide with improvement in migraine through shared pathophysiological mechanisms. Dietary changes and nutritional supplements also impact microbial ecology and gastrointestinal health and function, which may, at least in part, provide one mechanism by which they exert beneficial effects. Probiotics have limited evidence in migraine, with only one study of a specific multi-strain probiotic suggesting benefit, while another was not effective. Microbiome testing, although widely available, cannot identify probiotic treatment responders so a 10-week therapeutic trial could be considered.
Nutrient combinations
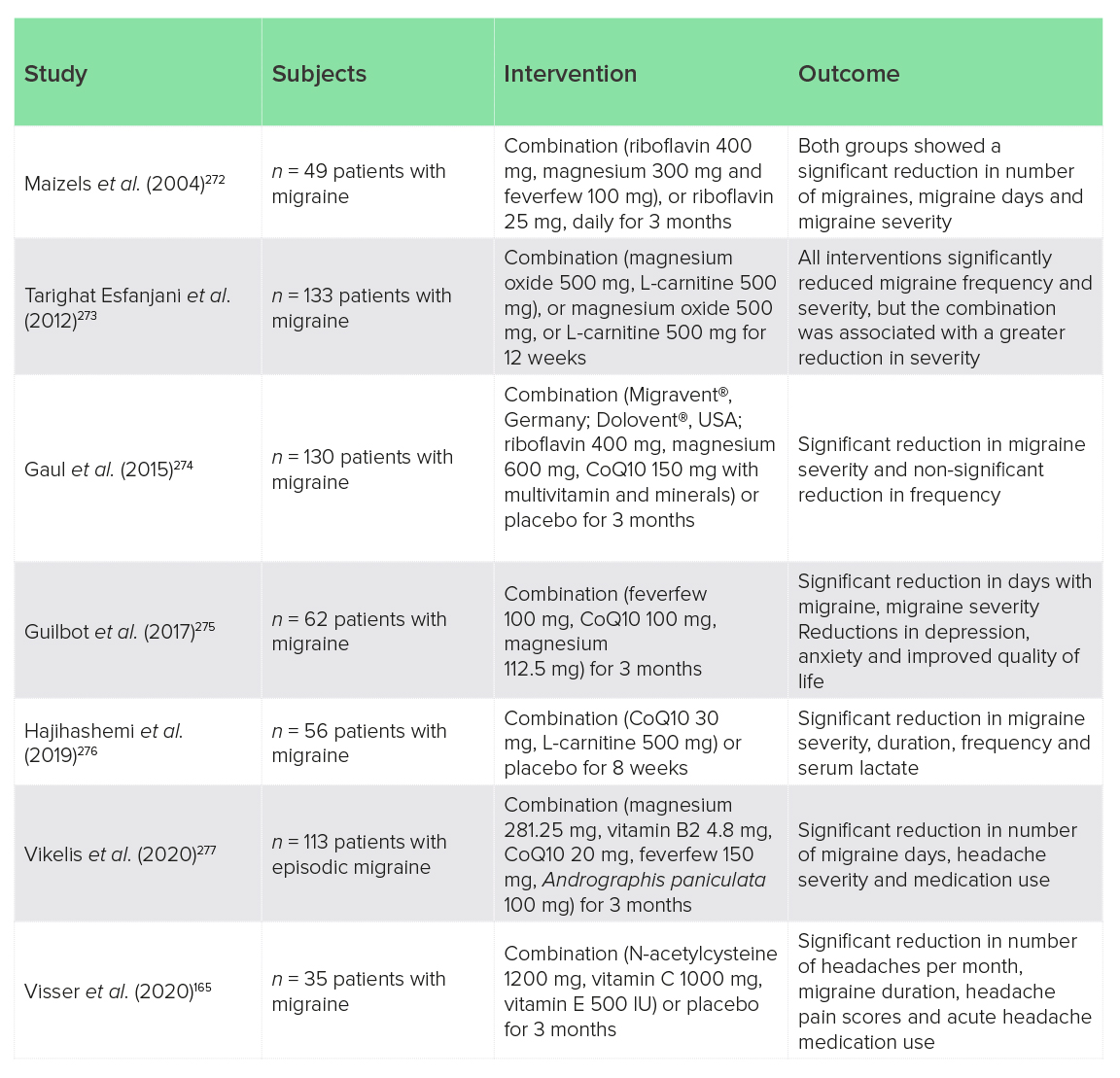
In addition to the monotherapies described above, some clinical trials have studied the effects of multi-component nutrient formulations (nutrient combinations). These are summarised below (Table 2). Most of these studies have suggested clinically relevant reductions in migraine frequency and severity, but are limited by lack of placebo control (four out of seven studies). Few compared nutrient combinations with individual nutrients to determine if there is an additive or synergistic advantage to a multi-component formula over individual components. Tarighat Esfanjani et al.273 did report a slightly better effect of magnesium and carnitine versus either nutrient alone, but Maizels et al.272 found that a relatively low dose of riboflavin that was intended as the control was as effective as a combination of high-dose riboflavin, magnesium and feverfew. And no studies personalised interventions to pathophysiological sub-groups, such as mitochondrial dysfunction or oxidative stress, or examined biological characteristics of treatment responders, which would be informative. Despite these limitations, nutrient combinations appear to have promise for the management of migraine.
Table 2: Clinical trials of nutrient combinations for migraine
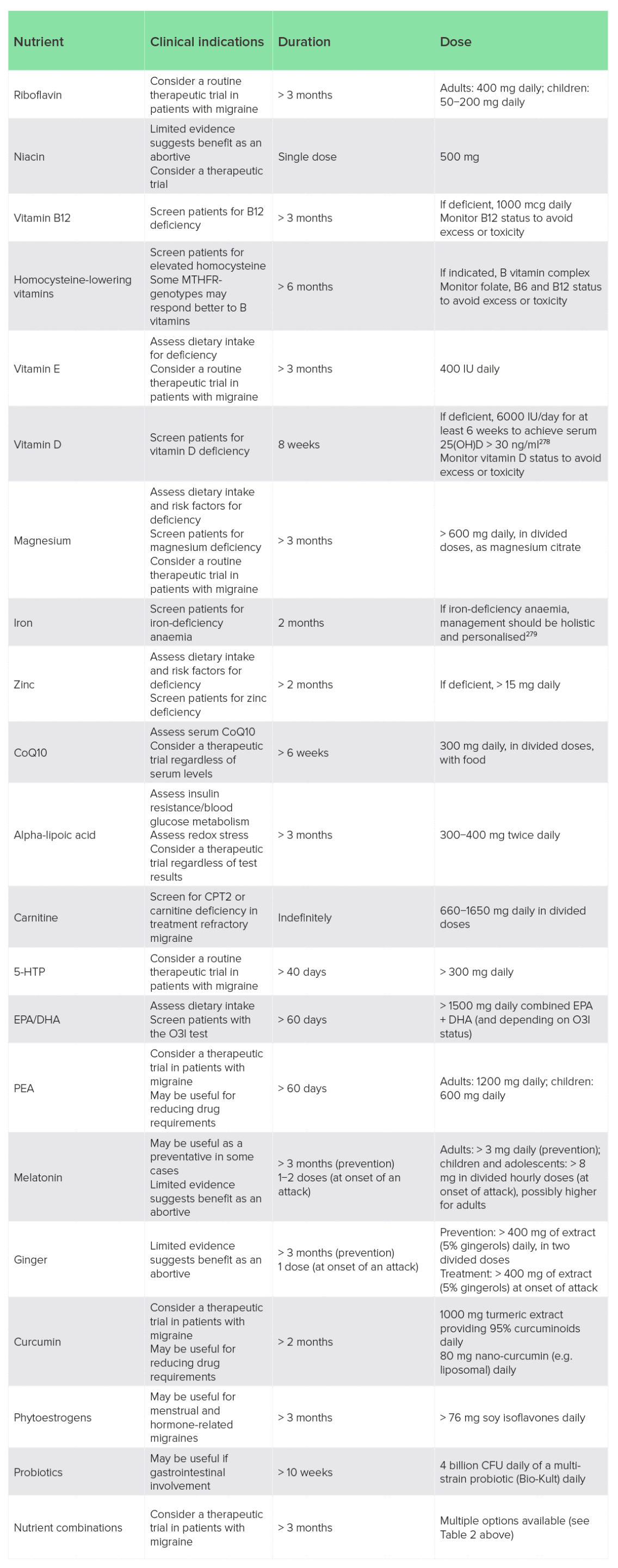
Table 3: Clinical summary of nutritional supplements for migraine
A wide range of nutritional interventions have been studied clinically and could be considered when managing migraine in clinical practice (Table 3). ‘Clinical indications’ include clinical history, symptoms and biomarkers that may help guide personalisation. Treatment duration and dose are based on clinical studies cited and discussed in this review, and are generally indicative of effective dose and time to see a significant clinical response. Importantly, clinical studies typically have indiscriminately tested interventions and have rarely utilised biomarkers or clinical symptoms to identify treatment responders, so the ‘clinical indications’ are in some cases based on the author’s opinion regarding the rationalisation of different management options.
Discussion
Migraine headaches have complex relationships to food and nutrition related to pathogenesis and opportunities for clinical management. Dietary interventions can be broadly divided into three major categories that may also be indications for their clinical use: healthy diet; identify aggravating foods or intolerances; and modify underlying pathophysiology or dysfunction. Clinical indications, such as dietary history, and biomarkers or investigations, such as the positive confirmation of coeliac disease, may help identify which dietary interventions are best suited to an individual (Figure 1).
Nutritional intervention with vitamins, minerals, amino acids and other nutrients have shown considerable promise for the management of migraine. A broad range of nutritional interventions is possible, so a pragmatic model that rationalises their indications and use may be helpful in clinical practice (Figure 2). Suboptimal intakes or deficiencies of vitamins, minerals and fatty acids can be assessed through dietary recall and/or laboratory testing, although both have limitations and may not accurately identify treatment responders, and nutrients may have pharmacological effects independent of deficiency or low dietary intake. Other nutrients have more specific indications like improving mitochondrial function, blood glucose metabolism, oestrogen stability or gut microbiome ecology; however, it should be emphasized that nutritional compounds have diverse mechanisms of action so this may be an oversimplification. Additionally, currently accessible laboratory testing may not be a reliable way to direct treatment, as in the cases of mitochondrial function, oestrogen stability and gut microbial ecology, for which there are few, if any, well-established clinical assessments relevant to migraine. Until reliable assessment tools are available, a therapeutic trial with a nutritional intervention, and subsequent positive or null symptomatic response, may be the most pragmatic assessment.
There is a need for clinical guidelines, treatment algorithms and a greater number of validated biomarkers that could help clinicians match nutritional and lifestyle medicine-based interventions to patients mostly likely to benefit.280 In the meantime, patient symptoms, health history, genetics, environment, lifestyle, diet and behaviour, amongst other considerations, can be leveraged in a clinical setting and inform personalised management plans.281 Nutritional approaches for migraine management can be used in a personalised and pragmatic way to improve patient outcomes.282
Conclusion
Chronic migraine headaches are a prevalent and debilitating disease that would benefit from better management options. A diverse range of therapeutic nutritional options have been explored for migraine headache, with varying degrees of benefit. The wide range of therapeutic options may make it challenging to approach nutritional management of migraine in a clinical setting, so a pragmatic model that helps personalise interventions from clinical signs and symptoms and reliable biomarkers would be useful. There is a need for clinical guidelines, treatment algorithms and a greater number of validated biomarkers that could help clinicians match nutritional and lifestyle-medicine-based interventions to patients most likely to respond to different interventions. Although a greater understanding of the role of nutritional medicine for migraine is needed, a considerable body of clinical evidence already exists, and could inform clinical practice in a way that improves patient outcomes and reduces suffering associated with the disease.
Acknowledgements
Author contributions: B. Brown carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: No funding was received for this work.
Declaration of interest: B. Brown has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA.
.
References
1 Younger, D. S. (2016) Epidemiology of migraine. Neurol. Clin., 34 (4), 849−861.
2 Natoli, J. L. et al. (2010) Global prevalence of chronic migraine: a systematic review. Cephalalgia, 30, 599–609.
3 Lipton, R. B., Bigal, M. E., Diamond, M., Freitag, F., Reed, M. L. & Stewart, W. F.; AMPP Advisory Group (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology, 68 (5), 343−349.
4 Ashina, M. et al. (2021) Migraine: disease characterisation, biomarkers, and precision medicine. Lancet, 397 (10 283), 1496−1504.
5 Karsan, N. & Goadsby, P. J. (2021) Migraine is more than just headache: is the link to chronic fatigue and mood disorders simply due to shared biological systems? Front. Hum. Neurosci., 15, 646 692.
6 Karsan, N. & Goadsby, P. J. (2018) Biological insights from the premonitory symptoms of migraine. Nat. Rev. Neurol., 14 (12), 699−710.
7 Carod-Artal, F. J. (2014) Tackling chronic migraine: current perspectives. J. Pain Res., 7, 185−194.
8 May, A. & Schulte, L. H. (2016) Chronic migraine: risk factors, mechanisms and treatment. Nat. Rev. Neurol., 12 (8), 455−464.
9 Rattanawong, W., Rapoport, A. & Srikiatkhachorn, A. (2022) Neurobiology of migraine progression. Neurobiol. Pain, 12, 100 094.
10 Goschorska, M., Gutowska, I., Baranowska-Bosiacka, I., Barczak, K. & Chlubek, D. (2020) The use of antioxidants in the treatment of migraine. Antioxidants (Basel), 9 (2), 116.
11 Lippi, G., Mattiuzzi, C. & Cervellin, G. (2014) C-reactive protein and migraine. Facts or speculations? Clin. Chem. Lab. Med., 52 (9), 1265−1272.
12 Rainero, I., Govone, F., Gai, A., Vacca, A. & Rubino, E. (2018) Is migraine primarily a metaboloendocrine disorder? Curr. Pain Headache Rep., 22 (5), 36.
13 Dodick, D. W. (2018) A phase-by-phase review of migraine pathophysiology. Headache, 58 (Suppl 1), 4−16.
14 Peck, K. R., Johnson, Y. L. & Smitherman, T. A. (2016) Migraine. Handb. Clin. Neurol., 138, 283−293.
15 Bigal, M., Krymchantowski, A. V. & Lipton, R. B. (2009) Barriers to satisfactory migraine outcomes. What have we learned, where do we stand? Headache, 49 (7), 1028−1041.
16 Sun-Edelstein, C. & Rapoport, A. M. (2016) Update on the pharmacological treatment of chronic migraine. Curr. Pain Headache Rep., 20 (1), 6.
17 Sheeler, R. D., Garza, I., Vargas, B. B. & O’Neil, A. E. (2016) Chronic daily headache: ten steps for primary care providers to regain control. Headache, 56 (10), 1675−1684.
18 Sun-Edelstein, C. & Mauskop, A. (2011) Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache, 51, 469–483.
19 Sun-Edelstein, C. & Mauskop, A. (2009) Foods and supplements in the management of migraine headaches. Clin. J. Pain, 25 (5), 446−452.
20 Robblee, J. & Starling, A. J. (2019) SEEDS for success: Lifestyle management in migraine. Cleve Clin. J. Med., 86 (11), 741−749.
21 Rajapakse, T. & Davenport, W. J. (2019) Phytomedicines in the treatment of migraine. CNS Drugs, 33 (5), 399−415.
22 Gazerani, P. (2020) Migraine and diet. Nutrients, 12 (6), 1658.
23 Razeghi Jahromi, S., Ghorbani, Z., Martelletti, P., Lampl, C. & Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS) (2019) Association of diet and headache. J. Headache Pain, 20 (1), 106.
24 Hajjarzadeh, S., Nikniaz, Z., Shalilahmadi, D., Mahdavi, R. & Behrouz, M. (2019) Comparison of diet quality between women with chronic and episodic migraine. Headache, 59 (8), 1221−1228.
25 Hajjarzadeh, S., Mahdavi, R., Shalilahmadi, D. & Nikniaz, Z. (2020) The association of dietary patterns with migraine attack frequency in migrainous women. Nutr. Neurosci., 23 (9), 724−730.
26 Khorsha, F. et al. (2020) Association between diet and migraine characteristics: The role of dietary inflammatory index. Curr. J. Neurol., 19 (2), 67–75.
27 Martins, L. B. et al. (2022) The quality and inflammatory index of the diet of patients with migraine. Nutr. Neurosci., 25 (10), 2092−2099.
28 Khorsha, F., Mirzababaei, A., Togha, M. & Mirzaei, K. (2021) Association of dietary diversity score (DDS) and migraine headache severity among women. Neurol. Sci., 42 (8), 3403−3410.
29 Khorsha, F., Mirzababaei, A., Togha, M. & Mirzaei, K. (2020) Association of drinking water and migraine headache severity. J. Clin. Neurosci., 77, 81−84.
30 Costa, A. B. P. et al. (2019) Nutritional intervention may improve migraine severity: a pilot study. Arq. Neuropsiquiatr., 77 (10), 723−730.
31 Altamura, C. et al. (2018) Promoting healthy eating can help preventing migraine: a real-life preliminary study. Neurol. Sci., 39 (Suppl 1), 155−156.
32 Altamura, C. et al. (2020) The healthy eating plate advice for migraine prevention: an interventional study. Nutrients, 12 (6), 1579.
33 Bic, Z., Blix, G. G., Hopp, H. P., Leslie, F. M. & Schell, M. J. (1999) The influence of a low-fat diet on incidence and severity of migraine headaches. J. Womens Health Gend. Based Med., 8 (5), 623−630.
34 Ferrara, L. A. et al. (2015) Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr. Metab. Cardiovasc. Dis., 25 (4), 370−375.
35 Perzia, B. M., Dunaief, J. L. & Dunaief, D. M. (2021) Chronic migraine reversal and prevention with the LIFE diet: a nutrient dense whole food plant-based diet (WFPBD). BMJ Case Rep., 14 (12), e243987.
36 Finocchi, C. & Sivori, G. (2012) Food as trigger and aggravating factor of migraine. Neurol. Sci., 33 (Suppl 1), S77−S80.
37 Zaeem, Z., Zhou, L. & Dilli, E. (2016) Headaches: a review of the role of dietary factors. Curr. Neurol. Neurosci. Rep., 16 (11), 101.
38 Rist, P. M., Buring, J. E. & Kurth, T. (2015) Dietary patterns according to headache and migraine status: a cross-sectional study. Cephalalgia, 35 (9), 767−775.
39 Pogoda, J. M.et al. (2016) Severe headache or migraine history is inversely correlated with dietary sodium intake: NHANES 1999−2004. Headache, 56 (4), 688−698.
40 Martin, V. T. & Vij, B. (2016) Diet and headache: Part 1. Headache, 56 (9), 1543−1552.
41 Gibb, C., Davies, P., Glover, V., Steiner, T., Rose, F. C. & Sandler, M. (1991) Chocolate is a migraine-provoking agent. Cephalalgia, 11, 93–95.
42 Marcus, D. A., Scharff, L., Turk, D. & Gourley, L. M. (1997) A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia, 17, 855–862.
43 Moffett, A. M., Swash, M. & Scott, D. F. (1974) Effect of chocolate in migraine: a double-blind study. J. Neurol. Neurosurg. Psychiatry, 37, 445–448.
44 Van den Eeden, S. K., Koepsell, T. D., Longstreth, W. T., van Belle, G., Daling, J. R. & McKnight, B. (1994) Aspartame ingestion and headaches: a randomized crossover trial. Neurology, 44, 1787–1793.
45 Koehler, S. M. & Glaros, A. (1988) The effect of aspartame on migraine headache. Headache, 28, 10–14.
46 Schiffman, S. S., Buckley, C. E. & Sampson, H. A. (1987) Aspartame and susceptibility to headache. N. Engl. J. Med., 317, 1181.
47 Sathyapalan, T. et al. (2015) Aspartame sensitivity? A double blind randomised crossover study. PLoS One, 10 (3), e0116212.
48 Özön, A. Ö., Karadaş, Ö. & Özge, A. (2016) Efficacy of diet restriction on migraines. Noro Psikiyatr Ars., 55 (3), 233−237.
49 Özön, A. Ö. & Karadaş, Ö. (2020) The effectiveness of diet restriction in elderly with migraine. Noro Psikiyatr Ars., 58 (3), 217−220.
50 Bunner, A. E., Agarwal, U., Gonzales, J. F., Valente, F. & Barnard, N. D. (2014) Nutrition intervention for migraine: a randomized crossover trial. J. Headache Pain, 15 (1), 69.
51 Cicarelli, G. et al. (2003) Clinical and neurological abnormalities in adult celiac disease. Neurol. Sci., 24, 311−317.
52 Briani, C. et al. (2008) Neurological complications of celiac disease and autoimmune mechanisms: A prospective study. J. Neuroimmunol., 195, 171−175.
53 Dimitrova, A. K. et al. (2013) Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache, 53, 344−355.
54 Fanaeian, M. M., Alibeik, N., Ganji, A., Fakheri, H., Ekhlasi, G. & Shahbazkhani, B. (2021) Prevalence of migraine in adults with celiac disease: A case control cross-sectional study. PLoS One, 16 (11), e0259502.
55 Hadjivassiliou, M., Grünewald, R. A. & Davies-Jones, G. A. (2002) Gluten sensitivity as a neurological illness. J. Neurol. Neurosurg. Psychiatry, 72 (5), 560−563.
56 Benjilali, L., Zahlane, M. & Essaadouni, L. (2012) A migraine as initial presentation of celiac disease. Rev. Neurol. (Paris), 168 (5), 454−456.
57 Hadjivassiliou, M. et al. (2001) Headache and CNS white matter abnormalities associated with gluten sensitivity. Neurology, 56, 385−388.
58 Gabrielli, M. et al. (2003) Association between migraine and Celiac disease: results from a preliminary case-control and therapeutic study. Am. J. Gastroenterol., 98 (3), 625−629.
59 Ameghino, L., Farez, M. F., Wilken, M. & Goicochea, M. T. (2019) Headache in patients with celiac disease and its response to the gluten-free diet. J. Oral Facial Pain Headache, 33 (3), 294–300.
60 Bürk, K. et al. (2009) Neurological symptoms in patients with biopsy proven celiac disease. Mov. Disord., 24 (16), 2358−2362.
61 Dimitrova, A. K. et al. (2013) Prevalence of migraine in patients with celiac disease and inflammatory bowel disease. Headache, 53 (2), 344−355.
62 Griauzdaitė, K. et al. (2020) Associations between migraine, celiac disease, non-celiac gluten sensitivity and activity of diamine oxidase. Med. Hypotheses, 142, 109 738.
63 Shahbazkhani, B. et al. (2020) Prevalence of non-celiac gluten sensitivity in patients with refractory functional dyspepsia: a randomized double-blind placebo controlled trial. Sci. Rep., 10 (1), 2401.
64 Gabrielli, M., Ojetti, V., Candelli, M., Santarelli, L., Gasbarrini, A. & Franceschi, F. (2021) Migraine as a common extra-intestinal presentation of celiac disease. OBM Neurobiol., 5 (1), 087. doi:10.21926/obm.neurobiol.2101087.
65 Catassi, C. et al. (2015) Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ Criteria. Nutrients, 7 (6), 4966−4977.
66 Geiselman, J. F. (2019) The clinical use of IgG food sensitivity testing with migraine headache patients: a literature review. Curr. Pain Headache Rep., 23 (11), 79.
67 Rees, T. & Watson, D. (2005) A prospective audit of food intolerance among migraine patients in primary care clinical practice. Headache Care, 2, 11–14.
68 Arroyave Hernandez, C. M., Echevarria Pinto, M. & Hernandez Montiel, H. L. (2007) Food allergy mediated by IgG 834 Cephalalgia 30(7) antibodies associated with migraine in adults. Rev. Alerg. Mex., 54, 162–168.
69 Alpay, K. et al. (2010) Diet restriction in migraine, based on IgG against foods: a clinical double-blind, randomised, cross-over trial. Cephalalgia, 30 (7), 829−837.
70 Mitchell, N. et al. (2011) Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr. J., 10, 85.
71 Cole, J. A., Rothman, K. J., Cabral, H. J., Zhang, Y. & Farraye, F. A. (2006) Migraine, fibromyalgia, and depression among people with IBS: a prevalence study. BMC Gastroenterol., 6, 26.
72 Brown, B. I. (2019) Does irritable bowel syndrome exist? Identifiable and treatable causes of associated symptoms suggest it may not. Gastrointest. Disord., 1 (3), 314−340.
73 Aydinlar, E. I. et al. (2013) IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache, 53 (3), 514−525.
74 Xie, Y. et al. (2019) Effects of diet based on IgG elimination combined with probiotics on migraine plus irritable bowel syndrome. Pain Res. Manag., 2019, 7890461. (1984)
75 Certain foods provoke migraine. Nutr. Rev., 42 (2), 41−42.
76 Monro, J., Carini, C. & Brostoff, J. (1984) Migraine is a food-allergic disease. Lancet, 2 (8405), 719−721.
77 Monro, J. A. (1983) Food allergy in migraine. Proc. Nutr. Soc., 42 (2), 241−246.
78 Egger, J., Carter, C. M., Wilson, J., Turner, M. W. & Soothill, J. F. (1983) Is migraine food allergy? A double-blind controlled trial of oligoantigenic diet treatment. Lancet, 2, 865–869.
79 Egger, J., Carter, C. M., Soothill, J. F. & Wilson, J. (1989) Oligoantigenic diet treatment of children with epilepsy and migraine. J. Pediatr., 114 (1), 51−58.
80 Egger, J., Carter, C. H., Soothill, J. F. & Wilson, J. (1992) Effect of diet treatment on enuresis in children with migraine or hyperkinetic behavior. Clin. Pediatr. (Phila.), 31 (5), 302−307.
81 Mansfield, L. E., Vaughan, T. R., Waller, S. F., Haverly, R. W. & Ting, S. (1985) Food allergy and adult migraine: double-blind and mediator confirmation of an allergic etiology. Ann. Allergy, 55 (2), 126−129.
82 Maintz, L. & Novak, N. (2007) Histamine and histamine intolerance. Am. J. Clin. Nutr., 85 (5), 1185−1196.
83 Kohn, J. B. (2014) Is there a diet for histamine intolerance? J. Acad. Nutr. Diet, 114 (11), 1860.
84 Schwelberger, H. G. (2010) Histamine intolerance: a metabolic disease? Inflamm. Res., 59 (Suppl 2), S219−S221.
85 García-Martín, E. et al. (2015) Diamine oxidase rs10156191 and rs2052129 variants are associated with the risk for migraine. Headache, 55 (2), 276−286.
86 Izquierdo-Casas, J. et al. (2018) Low serum diamine oxidase (DAO) activity levels in patients with migraine. J. Physiol. Biochem., 74 (1), 93−99.
87 Wantke, F., Götz, M. & Jarisch, R. (1994) The red wine provocation test: intolerance to histamine as a model for food intolerance. Allergy Proc., 15 (1), 27−32.
88 Izquierdo-Casas, J. et al. (2019) Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr., 38 (1), 152−158.
89 Reese, I. et al. (2021) Guideline on management of suspected adverse reactions to ingested histamine: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergology and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA) as well as the Swiss Society for Allergology and Immunology (SGAI) and the Austrian Society for Allergology and Immunology (ÖGAI). Allergol. Select., 5, 305−314.
90 Bond, D. S., Roth, J., Nash, J. M. & Wing, R. R. (2011) Migraine and obesity: epidemiology, possible mechanisms and the potential role of weight loss treatment. Obes. Rev., 12 (5), e362−e371.
91 Cervoni, C., Bond, D. S. & Seng, E. K. (2016) Behavioral weight loss treatments for individuals with migraine and obesity. Curr. Pain Headache Rep., 20 (2), 13.
92 Verrotti, A. et al. (2013) Impact of a weight loss program on migraine in obese adolescents. Eur. J. Neurol., 20 (2), 394−397.
93 Wilkinson, C. F. Jr (1949) Recurrent migrainoid headaches associated with spontaneous hypoglycemia. Am. J. Med. Sci., 218, 209–212.
94 Christensen, L. (1981) Hypoglycemia: implications and suggestions for research. Orthomol. Psychiat., 10, 77−92.
95 Dexter, J. D., Roberts, J. & Byer, J. A. (1978) The five hour glucose tolerance test and effect of low sucrose diet in migraine. Headache, 18 (2), 91−94.
96 Di Lorenzo, C. et al. (2015) Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur. J. Neurol., 22 (1), 170−177.
97 Kokavec, A. (2014) Dietary therapy could be an important factor in the prevention of headache symptoms in migraine (without aura): a case study. Food Public Health, 4 (2), 15−22.
98 Evcili, G., Utku, U., Öğün, M. N. & Özdemir, G. (2018) Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri., 30 (1), 8−11.
99 Simopoulos, A. P. (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr., 21 (6), 495−505.
100 Kalogeropoulos, N. et al. (2010) Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin. Chim. Acta, 411 (7−8), 584−591.
101 Ramsden, C. E. et al. (2013) Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain, 154 (11), 2441−2451.
102 Ramsden, C. E. et al. (2015) Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain, 156 (4), 587−596.
103 Ramsden, C. E. et al. (2021) Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: randomized controlled trial. BMJ, 374, n1448.
104 Pietrzak, D., Kasperek, K., Rękawek, P. & Piątkowska-Chmiel, I. (2022) The therapeutic role of ketogenic diet in neurological disorders. Nutrients, 14 (9), 1952.
105 Di Lorenzo, C. et al. (2016) Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J. Headache Pain, 17, 58.
106 Schnabel, T. G. (1928) An experience with a ketogenic dietary in migraine. Ann. Intern. Med., 2, 341−347. doi: 10.7326/0003-4819-2-4-341.
107 Barborka, C. J. (1930) Migraine: Results of treatment by ketogenic diet in fifty cases. J. Am. Med. Assoc., 95, 1825–1828.
108 Strahlman, R. S. (2006) Can ketosis help migraine sufferers? A case report. Headache, 46 (1), 182.
109 Di Lorenzo, C. et al. (2013) Diet transiently improves migraine in two twin sisters: possible role of ketogenesis? Funct. Neurol., 28 (4), 305−308.
110 Di Lorenzo, C., Coppola, G., Sirianni, G. & Pierelli, F. (2013) Short term improvement of migraine headaches during ketogenic diet: a prospective observational study in a dietician clinical setting. J. Headache Pain, 14 (Suppl 1), P219.
111 Di Lorenzo, C. et al. (2015) Migraine improvement during short lasting ketogenesis: a proof-of-concept study. Eur. J. Neurol., 22 (1), 170−177.
112 Di Lorenzo, C. et al. (2019) A randomized double-blind, cross-over trial of very low-calorie diet in overweight migraine patients: a possible role for ketones? Nutrients, 11 (8), 1742.
113 Haslam, R. L., Bezzina, A., Herbert, J., Spratt, N., Rollo, M. E. & Collins, C. E. (2021) Can ketogenic diet therapy improve migraine frequency, severity and duration? Healthcare (Basel), 9 (9), 1105.
114 Bongiovanni, D. et al. (2021) Effectiveness of ketogenic diet in treatment of patients with refractory chronic migraine. Neurol. Sci., 42 (9), 3865−3870.
115 Lovati, C., d’Alessandro, C. M., Ventura, S. D., Muzio, F. & Pantoni, L. (2022) Ketogenic diet in refractory migraine: possible efficacy and role of ketone bodies-a pilot experience. Neurol. Sci. [published online ahead of print, Aug 11]. 10.1007/s10072-022-06311-5.
116 Putananickal, N. et al. (2022) Efficacy and safety of exogenous beta-hydroxybutyrate for preventive treatment in episodic migraine: A single-centred, randomised, placebo-controlled, double-blind crossover trial. Cephalalgia, 42 (4−5), 302−311.
117 Wolkodoff, N. E., Haase, G. M. & Firger, R. A. (2020) The effects of a unique medium chain triglyceride complex on migraine symptoms: A beta pilot study. World J. Adv. Res. Rev., 8, 175−183. doi: 10.30574/wjarr.2020.8.3.0479.
118 Di Lorenzo, C. et al. (2021) Applications of ketogenic diets in patients with headache: clinical recommendations. Nutrients, 13 (7), 2307.
119 Marashly, E. T. & Bohlega, S. A. (2017) Riboflavin has neuroprotective potential: focus on Parkinson’s disease and migraine. Front. Neurol., 8, 333.
120 Thompson, D. F. & Saluja, H. S. (2017) Prophylaxis of migraine headaches with riboflavin: A systematic review. J. Clin. Pharm. Ther., 42 (4), 394−403. doi: 10.1111/jcpt.12548.
121 Condò, M., Posar, A., Arbizzani, A. & Parmeggiani, A. (2009) Riboflavin prophylaxis in pediatric and adolescent migraine. J. Headache Pain, 10 (5), 361−365. doi: 10.1007/s10194-009-0142-2.
122 Talebian, A., Soltani, B., Banafshe, H. R., Moosavi, G. A., Talebian, M. & Soltani, S. (2018) Prophylactic effect of riboflavin on pediatric migraine: a randomized, double-blind, placebo-controlled trial. Electron Physician, 10 (2), 6279−6285. doi: 10.19082/6279.
123 MacLennan, S. C., Wade, F. M., Forrest, K. M., Ratanayake, P. D., Fagan, E. & Antony, J. (2008) High-dose riboflavin for migraine prophylaxis in children: a double-blind, randomized, placebo-controlled trial. J. Child. Neurol., 23 (11), 1300−1304. doi: 10.1177/0883073808318053.
124 Bruijn, J., Duivenvoorden, H., Passchier, J., Locher, H., Dijkstra, N. & Arts, W. F. (2010) Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial. Cephalalgia, 30 (12), 1426−1434. doi: 10.1177/0333102410365106.
125 Condò, M., Posar, A., Arbizzani, A. & Parmeggiani, A. (2009) Riboflavin prophylaxis in pediatric and adolescent migraine. J. Headache Pain, 10 (5), 361−365. doi: 10.1007/s10194-009-0142-2.
126 Schoenen, J., Lenaerts, M. & Bastings, E. (1994) High-dose riboflavin as a prophylactic treatment of migraine: results of an open pilot study. Cephalalgia, 14 (5), 328−329. doi: 10.1046/j.1468-2982.1994.1405328.
127 Schoenen, J., Jacquy, J. & Lenaerts, M. (1998) Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology, 50 (2), 466−470. doi: 10.1212/wnl.50.2.466.
128 Rahimdel, A., Zeinali, A., Yazdian-Anari, P., Hajizadeh, R. & Arefnia, E. (2015) Effectiveness of vitamin B2 versus sodium valproate in migraine prophylaxis: a randomized clinical trial. Electron Physician, 7 (6), 1344−1348. doi: 10.14661/1344.
129 Boehnke, C., Reuter, U., Flach, U., Schuh-Hofer, S., Einhäupl, K. M. & Arnold, G. (2004) High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur. J. Neurol., 11 (7), 475−477. doi: 10.1111/j.1468-1331.2004.00813.
130 Yamanaka, G. et al. (2020) Effectiveness of low-dose riboflavin as a prophylactic agent in pediatric migraine. Brain Dev., 42 (7), 523−528. doi: 10.1016/j.braindev.2020.04.002.
131 Maizels, M., Blumenfeld, A. & Burchette, R. (2004) A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache, 44 (9), 885−890. doi: 10.1111/j.1526-4610.2004.04170.
132 Zempleni, J., Galloway, J. R. & McCormick, D. B. (1996) Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am. J. Clin. Nutr., 63 (1), 54−66. doi: 10.1093/ajcn/63.1.54.
133 Saedisomeolia, A. & Ashoori, M. (2018) Riboflavin in human health: a review of current evidences. Adv. Food Nutr. Res., 83, 57−81. doi: 10.1016/bs.afnr.2017.11.002.
134 Di Lorenzo, C. et al. (2009) Mitochondrial DNA haplogroups influence the therapeutic response to riboflavin in migraineurs. Neurology, 72 (18), 1588−1594. doi: 10.1212/WNL.0b013e3181a41269.
135 Ames, B. N., Elson-Schwab, I. & Silver, E. A. (2002) High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am. J. Clin. Nutr., 75 (4), 616−658.
136 Prousky, J. & Seely, D. (2005) The treatment of migraines and tension-type headaches with intravenous and oral niacin (nicotinic acid): systematic review of the literature. Nutr. J., 4, 3. https://doi.org/10.1186/1475-2891-4-3.
137 Prousky, J., Millman, C. G. & Kirkland, J. B. (2011) Pharmacologic use of niacin. J. Evid. Based Complement. Alternat. Med., 16 (2), 91−101. doi: 10.1177/2156587211399579.
138 Prousky, J. & Sykes, E. (2003) Two case reports on the treatment of acute migraine with niacin. Its hypothetical mechanism of action upon calcitonin-gene related peptide and platelets. J. Orthomol. Med., 18, 108−110.
139 Velling, D. A., Dodick, D. W. & Muir, J. J. (2003) Sustained-release niacin for prevention of migraine headache. Mayo Clin. Proc., 78 (6), 770−771. doi: 10.4065/78.6.770.
140 Prousky, J. & Seely, D. (2005) The treatment of migraines and tension-type headaches with intravenous and oral niacin (nicotinic acid): systematic review of the literature. Nutr. J., 4, 3.
141 Peracchi, M., Bamonti Catena, F., Pomati, M., De Franceschi, M. & Scalabrino, G. (2001) Human cobalamin deficiency: alterations in serum tumour necrosis factor-alpha and epidermal growth factor. Eur. J. Haematol., 67 (2), 123−127.
142 van de Lagemaat, E. E., de Groot, L. C. P. G. M. & van den Heuvel, E. G. H. M. (2019) Vitamin B12 in relation to oxidative stress: a systematic review. Nutrients, 11 (2), 482.
143 Togha, M., Razeghi Jahromi, S., Ghorbani, Z., Martami, F. & Seifishahpar, M. (2019) Serum vitamin B12 and methylmalonic acid status in migraineurs: a case-control study. Headache, 59 (9), 1492−1503.
144 van Oijen, M. G., Laheij, R. J., Peters, W. H., Jansen, J. B. & Verheugt, F. W.; BACH study (2004) Association of aspirin use with vitamin B12 deficiency (results of the BACH study). Am. J. Cardiol., 94 (7), 975−977. doi: 10.1016/j.amjcard.2004.06.047.
145 Urits, I. et al. (2020) Utilization of B12 for the treatment of chronic migraine. Best Pract. Res. Clin. Anaesthesiol., 34 (3), 479−491.
146 van der Kuy, P. H., Merkus, F. W., Lohman, J. J., ter Berg, J. W. & Hooymans, P. M. (2002) Hydroxocobalamin, a nitric oxide scavenger, in the prophylaxis of migraine: an open, pilot study. Cephalalgia, 22 (7), 513−519. doi: 10.1046/j.1468-2982.2002.00412.
147 Calik, M. et al. (2018) The association between serum vitamin B12 deficiency and tension-type headache in Turkish children. Neurol. Sci., 39 (6), 1009−1014. doi: 10.1007/s10072-018-3286-5.
148 Rainero, I., Vacca, A., Roveta, F., Govone, F., Gai, A. & Rubino, E. (2019) Targeting MTHFR for the treatment of migraines. Expert Opin. Ther. Targets, 23 (1), 29−37.
149 Liampas, I. et al. (2020) Serum homocysteine, pyridoxine, folate, and vitamin B12 levels in migraine: systematic review and meta-analysis. Headache, 60 (8), 1508−1534.
150 Liu, L., Yu, Y., He, J., Guo, L., Li, H. & Teng, J. (2019) Effects of MTHFR C677T and A1298C polymorphisms on migraine susceptibility: a meta-analysis of 26 studies. Headache, 59 (6), 891−905. doi: 10.1111/head.13540.
151 Liampas, I. N. et al. (2020) Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol. Scand., 142 (2), 108−120. doi: 10.1111/ane.13251.
152 Di Rosa, G. et al.. (2007) Efficacy of folic acid in children with migraine, hyperhomocysteinemia and MTHFR polymorphisms. Headache, 47 (9), 1342−1344.
153 Lea, R., Colson, N., Quinlan, S., Macmillan, J. & Griffiths, L. (2009) The effects of vitamin supplementation and MTHFR (C677T) genotype on homocysteine-lowering and migraine disability. Pharmacogenet. Genomics, 19 (6), 422−428.
154 Menon, S. et al. (2012) Genotypes of the MTHFR C677T and MTRR A66G genes act independently to reduce migraine disability in response to vitamin supplementation. Pharmacogenet. Genomics, 22 (10), 741−749.
155 Menon, S. et al. (2015) Effects of dietary folate intake on migraine disability and frequency. Headache, 55 (2), 301−309. doi: 10.1111/head.12490.
156 Bütün, A., Nazıroğlu, M., Demirci, S., Çelik, Ö. & Uğuz, A. C. (2015) Riboflavin and vitamin E increase brain calcium and antioxidants, and microsomal calcium-ATP-ase values in rat headache models induced by glyceryl trinitrate. J. Membr. Biol., 248 (2), 205−213.
157 Ziaei, S., Kazemnejad, A. & Sedighi, A. (2009) The effect of vitamin E on the treatment of menstrual migraine. Med. Sci. Monit., 15 (1), CR16−CR19.
158 Visser, E. J., Drummond, P. D. & Lee-Visser, J. L. A. (2020) Reduction in migraine and headache frequency and intensity with combined antioxidant prophylaxis (N-acetylcysteine, vitamin E, and vitamin C): a randomized sham-controlled pilot study. Pain Pract., 20 (7), 737−747.
159 Ghorbani, Z. et al. (2019) Vitamin D in migraine headache: a comprehensive review on literature. Neurol. Sci., 40 (12), 2459−2477. doi: 10.1007/s10072-019-04021-z.
160 Ghorbani, Z. et al. (2021) The effects of vitamin D3 supplementation on TGF-β and IL-17 serum levels in migraineurs: post hoc analysis of a randomized clinical trial. J. Pharm. Health Care Sci., 7 (1), 9.
161 Ghorbani, Z. et al. (2020) The effects of vitamin D supplementation on interictal serum levels of calcitonin gene-related peptide (CGRP) in episodic migraine patients: post hoc analysis of a randomized double-blind placebo-controlled trial. J. Headache Pain, 21 (1), 22.
162 Ghorbani, Z. et al. (2020) Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: a randomized clinical trial. Neurol. Sci., 41 (5), 1183−1192.
163 Nowaczewska, M., Wiciński, M., Osiński, S. & Kaźmierczak, H. (2020) The role of vitamin D in primary headache-from potential mechanism to treatment. Nutrients, 12 (1), 243. doi: 10.3390/nu12010243.
164 Cayir, A., Turan, M. I. & Tan, H. (2014) Effect of vitamin D therapy in addition to amitriptyline on migraine attacks in pediatric patients. Braz. J. Med. Biol. Res., 47 (4), 349−354. doi: 10.1590/1414-431×20143606.
165 Gazerani, P. et al. (2019) A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D3 supplementation in adult patients with migraine. Curr. Med. Res. Opin., 35 (4), 715−723. doi: 10.1080/03007995.2018.1519503.
166 Siniscalchi, A., Lochner, P., Cione, E., Michniewicz, A., Guidetti, V. & Gallelli, L. (2019) Improved efficacy of pregabalin by restoring plasma vitamin D levels in migraine: a case report. Psychopharmacol. Bull., 49 (2), 41−45.
167 Dolati, S., Rikhtegar, R., Mehdizadeh, A. & Yousefi, M. (2020) The role of magnesium in pathophysiology and migraine treatment. Biol. Trace Elem. Res., 196 (2), 375−383. doi: 10.1007/s12011-019-01931-z.
168 Kirkland, A. E., Sarlo, G. L. & Holton, K. F. (2018) The role of magnesium in neurological disorders. Nutrients, 10 (6), 730. doi: 10.3390/nu10060730.
169 Ramadan, N. M., Halvorson, H., Vande-Linde, A., Levine, S. R., Helpern, J. A. & Welch, K. M. (1989) Low brain magnesium in migraine. Headache, 29 (9), 590−593. doi: 10.1111/j.1526-4610.1989.hed2909590.
170 Sarchielli, P., Coata, G., Firenze, C., Morucci, P., Abbritti, G. & Gallai, V. (1992) Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia, 12 (1), 21−27.
171 Assarzadegan, F., Asgarzadeh, S., Hatamabadi, H. R., Shahrami, A., Tabatabaey, A. & Asgarzadeh, M. (2016) Serum concentration of magnesium as an independent risk factor in migraine attacks: a matched case-control study and review of the literature. Int. Clin. Psychopharmacol., 31 (5), 287−292.
172 Slavin, M., Li, H., Khatri, M. & Frankenfeld, C. (2021) Dietary magnesium and migraine in adults: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2001−2004. Headache, 61 (2), 276−286.
173 von Luckner, A. & Riederer, F. (2018) Magnesium in migraine prophylaxis-is there an evidence-based rationale? A systematic review. Headache, 58 (2), 199−209.
174 Demirkaya, S., Vural, O., Dora, B. & Topçuoğlu, M. A. (2001) Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache, 41 (2), 171−177.
175 Mauskop, A. & Varughese, J. (2012) Why all migraine patients should be treated with magnesium. J. Neural Transm. (Vienna), 119 (5), 575−579.
176 Baratloo, A., Mirbaha, S., Delavar Kasmaei, H., Payandemehr, P., Elmaraezy, A. & Negida, A. (2017) Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J. Pain, 30 (3), 176−182.
177 Kandil, M. et al. (2021) MAGraine: Magnesium compared to conventional therapy for treatment of migraines. Am. J. Emerg. Med., 39, 28−33.
178 Pamuk, G. E. et al. (2016) Is iron-deficiency anemia associated with migraine? Is there a role for anxiety and depression? Wien Klin Wochenschr., 128 (Suppl 8), 576−580. doi: 10.1007/s00508-015-0740-8.
179 Tayyebi, A., Poursadeghfard, M., Nazeri, M. & Pousadeghfard, T. (2019) Is there any correlation between migraine attacks and iron deficiency anemia? A case-control study. Int. J. Hematol. Oncol. Stem Cell Res., 13 (3), 164−171.
180 Vuković-Cvetković, V., Plavec, D., Lovrencić-Huzjan, A., Galinović, I., Serić, V. & Demarin, V. (2010) Is iron deficiency anemia related to menstrual migraine? Post hoc analysis of an observational study evaluating clinical characteristics of patients with menstrual migraine. Acta Clin. Croat., 49 (4), 389−394.
181 Gür-Özmen, S. & Karahan-Özcan, R. (2016) Iron deficiency anemia is associated with menstrual migraine: a case-control study. Pain Med., 17 (3), 596−605. doi: 10.1093/pm/pnv029.
182 Fallah, R., Zare Bidoki, S. & Ordooei, M. (2016) Evaluation efficacy of ferrous sulfate therapy on headaches of 5-15 years old iron deficient children with migraine. Iran J. Ped. Hematol. Oncol., 6 (1), 32−37.
183 Gholamreza-Mirzaee, M., Kheiri, S., Khosravi, Sh., Koshdel, A., Keyvani, Z. & Amini, Z. (2012) Iron therapy and migraine headache. J. Shahrekord Univ. Med. Sci., 13 (6), 56–62 [in Persian].
184 Meng, S. H. et al. (2021) Association between dietary iron intake and serum ferritin and severe headache or migraine. Front. Nutr., 8, 685 564.
185 Gonullu, H. et al. (2015) The levels of trace elements and heavy metals in patients with acute migraine headache. J. Pak. Med. Assoc., 65 (7), 694−697.
186 Silva, M. L. et al. (2022) Decreased plasma levels and dietary intake of minerals in women with migraine. Nutr. Neurosci., 2022, 1−8 [published online ahead of print, Jun 3].
187 Liu, H. Y., Gale, J. R., Reynolds, I. J., Weiss, J. H. & Aizenman, E. (2021) The multifaceted roles of zinc in neuronal mitochondrial dysfunction. Biomedicines, 9 (5), 489.
188 Ahmadi, H. et al. (2020) Zinc supplementation affects favorably the frequency of migraine attacks: a double-blind randomized placebo-controlled clinical trial. Nutr. J., 19 (1), 101.
189 Mazaheri, M., Aghdam, A. M., Heidari, M. & Zarrin, R. (2021) Assessing the effect of zinc supplementation on the frequency of migraine attack, duration, severity, lipid profile and hs-CRP in adult women. Clin. Nutr. Res., 10 (2), 127−139.
190 Hershey, A. D. et al. (2007) Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. Headache, 47 (1), 73−80. doi: 10.1111/j.1526-4610.2007.00652.
191 Quinzii, C. M. et al. (2010) Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J., 24 (10), 3733−3743.
192 Dahri, M., Tarighat-Esfanjani, A., Asghari-Jafarabadi, M. & Hashemilar, M. (2019) Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci., 22 (9), 607−615.
193 Sazali, S., Badrin, S., Norhayati, M. N. & Idris, N. S. (2021) Coenzyme Q10 supplementation for prophylaxis in adult patients with migraine-a meta-analysis. BMJ Open, 11 (1), e039358. doi: 10.1136/bmjopen-2020-039358.
194 Parohan, M., Sarraf, P., Javanbakht, M. H., Ranji-Burachaloo, S. & Djalali, M. (2020) Effect of coenzyme Q10 supplementation on clinical features of migraine: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. Neurosci., 23 (11), 868−875.
195 Parohan, M., Sarraf, P., Javanbakht, M. H., Foroushani, A. R., Ranji-Burachaloo, S. & Djalali, M. (2021) The synergistic effects of nano-curcumin and coenzyme Q10 supplementation in migraine prophylaxis: a randomized, placebo-controlled, double-blind trial. Nutr. Neurosci., 24 (4), 317−326. doi: 10.1080/1028415X.2019.1627770.
196 Gross, E. C., Lisicki, M., Fischer, D., Sándor, P. S. & Schoenen, J. (2019) The metabolic face of migraine − from pathophysiology to treatment. Nat. Rev. Neurol., 15 (11), 627−643.
197 Cavestro, C., Bedogni, G., Molinari, F., Mandrino, S., Rota, E. & Frigeri, M. C. (2018) Alpha-lipoic acid shows promise to improve migraine in patients with insulin resistance: a 6-month exploratory study. J. Med. Food, 21 (3), 269−273.
198 Rezaei Kelishadi, M., Alavi Naeini, A., Askari, G., Khorvash, F. & Heidari, Z. (2021) The efficacy of alpha-lipoic acid in improving oxidative, inflammatory, and mood status in women with episodic migraine in a randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract., 75 (9), e14455.
199 Kelishadi, M. R., Naeini, A. A., Khorvash, F., Askari, G. & Heidari, Z. (2022) The beneficial effect of alpha-lipoic acid supplementation as a potential adjunct treatment in episodic migraines. Sci. Rep., 12 (1), 271.
200 Magis, D., Ambrosini, A., Sándor, P., Jacquy, J., Laloux, P. & Schoenen, J. (2007) A randomized double-blind placebo-controlled trial of thioctic acid in migraine prophylaxis. Headache, 47, 52−57.
201 Ali, A. M., Awad, T. G. & Al-Adl, N. M. (2010) Efficacy of combined topiramate/thioctic acid therapy in migraine prophylaxis. Saudi Pharm. J., 18, 239−243.
202 Traina, G. (2016) The neurobiology of acetyl-L-carnitine. Front. Biosci. (Landmark Ed.), 21 (7), 1314−1329. doi:10.2741/4459
203 Hagen, K. et al. (2015) Acetyl-l-carnitine versus placebo for migraine prophylaxis: A randomized, triple-blind, crossover study. Cephalalgia, 35 (11), 987−995.
204 Amini, L., Yaghini, O., Ghazavi, M. & Aslani, N. (2021) L-Carnitine versus propranolol for pediatric migraine prophylaxis. Iran. J. Child. Neurol., 15 (2), 77−86.
205 Kabbouche, M. A., Powers, S. W., Vockell, A. L., LeCates, S. L. & Hershey, A. D. (2003) Carnitine palmityltransferase II (CPT2) deficiency and migraine headache: two case reports. Headache, 43 (5), 490−495.
206 Hsu, C. C. et al. (1995) CPEO and carnitine deficiency overlapping in MELAS syndrome. Acta Neurol. Scand., 92 (3), 252−255.
207 Charleston, L. 4th, Khalil, S. & Young, W. B. (2021) Carnitine responsive migraine headache syndrome: case report and review of the literature. Curr. Pain Headache Rep., 25 (4), 26.
208 Panconesi, A. (2008) Serotonin and migraine: a reconsideration of the central theory. J. Headache Pain, 9 (5), 267−276.
209 Genazzani, A. R. et al. (1986) Effects of L-5HTP with and without carbidopa on plasma beta-endorphin and pain perception. Possible implications in migraine prophylaxis. Cephalalgia, 6 (4), 241−245.
210 Chauvel, V., Multon, S. & Schoenen, J. (2018) Estrogen-dependent effects of 5-hydroxytryptophan on cortical spreading depression in rat: Modelling the serotonin-ovarian hormone interaction in migraine aura. Cephalalgia, 38 (3), 427−436.
211 Turner, E. H., Loftis, J. M. & Blackwell, A. D. (2006) Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther., 109 (3), 325−338.
212 Sicuteri, F. (1972) 5-Hydroxytryptophan in the prophylaxis of migraine. Pharmacol. Res. Comm., 4 (3), 213−218.
213 De Benedittis, G. & Massei, R. (1985) Serotonin precursors in chronic primary headache. A double-blind cross-over study with L-5-hydroxytryptophan vs. placebo. J. Neurosurg. Sci., 29 (3), 239−248.
214 Titus, F., Dávalos, A., Alom, J. & Codina, A. (1986) 5-Hydroxytryptophan versus methysergide in the prophylaxis of migraine. Randomized clinical trial. Eur. Neurol., 25 (5), 327−329.
215 Santucci, M., Cortelli, P., Rossi, P. G., Baruzzi, A. & Sacquegna, T. (1986) L-5-hydroxytryptophan versus placebo in childhood migraine prophylaxis: a double-blind crossover study. Cephalalgia, 6 (3), 155−157.
216 De Giorgis, G., Miletto, R., Iannuccelli, M., Camuffo, M. & Scerni, S. (1987) Headache in association with sleep disorders in children: a psychodiagnostic evaluation and controlled clinical study−L-5-HTP versus placebo. Drugs Exp. Clin. Res., 13 (7), 425−433.
217 Nicolodi, M. & Sicuteri, F. (1996) Fibromyalgia and migraine, two faces of the same mechanism. Serotonin as the common clue for pathogenesis and therapy. Adv. Exp. Med. Biol., 398, 373−379.
218 De Benedittis, G. & Massei, R. (1985) Serotonin precursors in chronic primary headache. A double-blind cross-over study with L-5-hydroxytryptophan vs. placebo. J. Neurosurg. Sci., 29 (3), 239−248.
219 Ribeiro, C. A. (2000) L-5-Hydroxytryptophan in the prophylaxis of chronic tension-type headache: a double-blind, randomized, placebo-controlled study. For the Portuguese Head Society. Headache, 40 (6), 451−456.
220 Sanders, A. E., Shaikh, S. R. & Slade, G. D. (2018) Long-chain omega-3 fatty acids and headache in the U.S. population. Prostaglandins Leukot. Essent. Fatty Acids, 135, 47−53.
221 Sadeghi, O., Maghsoudi, Z., Khorvash, F., Ghiasvand, R. & Askari, G. (2015) The relationship between different fatty acids intake and frequency of migraine attacks. Iran. J. Nurs. Midwifery Res., 20 (3), 334−339.
222 von Schacky, C. (2021) Importance of EPA and DHA blood levels in brain structure and function. Nutrients, 13 (4), 1074. doi:10.3390/nu13041074.
223 Maghsoumi-Norouzabad, L., Mansoori, A., Abed, R. & Shishehbor, F. (2018) Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: a systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci., 21 (9), 614–623.
224 Soares, A. A., Louçana, P. M. C., Nasi, E. P., Sousa, K. M. H., Sá, O. M. S. & Silva-Néto, R. P. (2018) A double-blind, randomized, and placebo-controlled clinical trial with omega-3 polyunsaturated fatty acids (OPFA ɷ-3) for the prevention of migraine in chronic migraine patients using amitriptyline. Nutr. Neurosci., 21 (3), 219−223.
225 Guu, T. W. et al. (2019) International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother. Psychosom., 88 (5), 263−273.
226 Clayton, P., Hill, M., Bogoda, N., Subah, S. & Venkatesh, R. (2021) Palmitoylethanolamide: a natural compound for health management. Int. J. Mol. Sci., 22 (10), 5305.
227 Greco, R. et al. (2022) The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain. Headache, 62 (3), 227−240.
228 Gabrielsson, L., Mattsson, S. & Fowler, C. J. (2016) Palmitoylethanolamide for the treatment of pain: pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol., 82 (4), 932−942.
229 Chirchiglia, D. et al. (2018) Effects of add-on ultramicronized N-palmitol ethanol amide in patients suffering of migraine with aura: a pilot study. Front. Neurol., 9, 674.
230 Hernández, A. G. (2022) Palmitoylethanolamide-based nutraceutical Calmux® in preventive treatment of migraine. Clin. Neurol. Neurosurg., 218, 107 282.
231 Papetti, L. et al. (2020) Tolerability of palmitoylethanolamide in a pediatric population suffering from migraine: a pilot study. Pain Res. Manag., 2020, 3938640.
232 Andersen, L. P., Gögenur, I., Rosenberg, J. & Reiter, R. J. (2016) The safety of melatonin in humans. Clin. Drug Investig., 36 (3), 169−175.
233 Kozak, H. H. et al. (2017) Sleep quality, morningness-eveningness preference, mood profile, and levels of serum melatonin in migraine patients: a case-control study. Acta Neurol. Belg., 117 (1), 111−119.
234 Zielonka, D. (2021) Melatonin secretion in migraine patients: the current state of knowledge. Neurol. Neurochir. Pol., 55 (1), 5−7.
235 Rios, E. R. et al. (2010) Melatonin: pharmacological aspects and clinical trends. Int. J. Neurosci., 120 (9), 583−590.
236 Sullivan, D. P. (2022) Furthering the understanding of behavioral aspects of sleep and headaches: another piece of the puzzle. Sleep, 45 (5), zsac012.
237 Aaltonen, K., Hämäläinen, M. L. & Hoppu, K. (2000) Migraine attacks and sleep in children. Cephalalgia, 20 (6), 580−584.
238 Long, R., Zhu, Y. & Zhou, S. (2019) Therapeutic role of melatonin in migraine prophylaxis: A systematic review. Medicine (Baltimore), 98 (3), e14099.
239 Gelfand, A. A., Ross, A. C., Irwin, S. L., Greene, K. A., Qubty, W. F. & Allen, I. E. (2020) Melatonin for acute treatment of migraine in children and adolescents: a pilot randomized trial. Headache, 60 (8), 1712−1721.
240 Crichton, M. et al. (2022) Orally consumed ginger and human health: an umbrella review. Am. J. Clin. Nutr., 115 (6), 1511−1527.
241 Chen, L. & Cai, Z. (2021) The efficacy of ginger for the treatment of migraine: A meta-analysis of randomized controlled studies. Am. J. Emerg. Med., 46, 567−571.
242 Martins, L. B. et al. (2020) Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia, 40 (1), 88−95.
243 Cady, R. K., Goldstein, J., Nett, R., Mitchell, R., Beach, M. E. & Browning, R. (2011) A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic™ M) in the treatment of migraine. Headache, 51 (7), 1078−1086.
244 Martins, L. B., Rodrigues, A. M. D. S., Rodrigues, D. F., Dos Santos, L. C., Teixeira, A. L. & Ferreira, A. V. M. (2019) Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) addition in migraine acute treatment. Cephalalgia, 39 (1), 68−76. doi: 10.1177/0333102418776016.
245 Sedighiyan, M. et al. (2022) The effects of nano-curcumin supplementation on leptin and adiponectin in migraine patients: a double-blind clinical trial study from gene expression to clinical symptoms. Endocr. Metab. Immune Disord. Drug Targets [published online ahead of print, Jul 1]. 10.2174/1871530322666220701100817.
246 Djalali, M., Abdolahi, M., Hosseini, R., Miraghajani, M., Mohammadi, H. & Djalali, M. (2021) The effects of nano-curcumin supplementation on Th2/tregulatory axis in migraine patients: a randomized, double-blind, placebo-controlled trial. Int. J. Neurosci., 2021, 1−7.
247 Djalali, M., Abdolahi, M., Hosseini, R., Miraghajani, M., Mohammadi, H. & Djalali, M. (2020) The effects of nano-curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complement. Ther. Clin. Pract., 41, 101 256.
248 Rezaie, S., Askari, G., Khorvash, F., Tarrahi, M. J. & Amani, R. (2021) Effects of curcumin supplementation on clinical features and inflammation, in migraine patients: a double-blind controlled, placebo randomized clinical trial. Int. J. Prev. Med., 12, 161.
249 Abdolahi, M. et al. (2017) The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics, 69 (6), 371−378.
250 Abdolahi, M. et al. (2018) A novel combination of ω-3 fatty acids and nano-curcumin modulates interleukin-6 gene expression and high sensitivity C-reactive protein serum levels in patients with migraine: a randomized clinical trial study. CNS Neurol. Disord. Drug Targets, 17 (6), 430−438.
251 Soveyd, N. et al. (2018) The combined effects of ω-3 fatty acids and nano-curcumin supplementation on intercellular adhesion molecule-1 (ICAM-1) gene expression and serum levels in migraine patients. CNS Neurol. Disord. Drug Targets, 16 (10), 1120−1126.
252 Honarvar, N. M., Soveid, N., Abdolahi, M., Djalali, M., Hatami, M. & Karzar, N. H. (2021) Anti-neuroinflammatory properties of n-3 fatty acids and nano-curcumin on migraine patients from cellular to clinical insight: a randomized, double-blind and placebo-controlled trial. Endocr. Metab. Immune Disord. Drug Targets, 21 (2), 365−373.
253 Karthikeyan, A., Senthil, N. & Min, T. (2020) Nanocurcumin: a promising candidate for therapeutic applications. Front. Pharmacol., 11, 487.
254 Delaruelle, Z. et al. (2018) Male and female sex hormones in primary headaches. J. Headache Pain, 19 (1), 117.
255 Ornello, R., De Matteis, E., Di Felice, C., Caponnetto, V., Pistoia, F. & Sacco, S. (2021) Acute and preventive management of migraine during menstruation and menopause. J. Clin. Med., 10 (11), 2263.
256 Pavlović, J. M. et al. (2016) Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology, 87 (1), 49−56.
257 Shuster, L. T., Faubion, S. S., Sood, R. & Casey, P. M. (2011) Hormonal manipulation strategies in the management of menstrual migraine and other hormonally related headaches. Curr. Neurol. Neurosci. Rep., 11 (2), 131−138.
258 Molla, M. D., Hidalgo-Mora, J. J. & Soteras, M. G. (2011) Phytotherapy as alternative to hormone replacement therapy. Front. Biosci. (Schol. Ed.), 3 (1), 191−204.
259 Burke, B. E., Olson, R. D. & Cusack, B. J. (2002) Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraine. Biomed. Pharmacother., 56 (6), 283−288.
260 Ferrante, F., Fusco, E., Calabresi, P. & Cupini, L. M. (2004) Phyto-oestrogens in the prophylaxis of menstrual migraine. Clin. Neuropharmacol., 27 (3), 137−140.
261 Dzator, J. S. A., Howe, P. R. C., Coupland, K. G. & Wong, R. H. X. (2022) A randomised, double-blind, placebo-controlled crossover trial of resveratrol supplementation for prophylaxis of hormonal migraine. Nutrients, 14 (9), 1763.
262 Cámara-Lemarroy, C. R., Rodriguez-Gutierrez, R., Monreal-Robles, R. & Marfil-Rivera, A. (2016) Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol., 22 (36), 8149−8160.
263 Gonzalez, A., Hyde, E., Sangwan, N., Gilbert, J. A., Viirre, E. & Knight, R. (2016) Migraines are correlated with higher levels of nitrate-, nitrite-, and nitric oxide-reducing oral microbes in the American Gut Project Cohort. mSystems, 1 (5), e00105-16.
264 Gasbarrini, A. et al. (2000) Association between Helicobacter pylori cytotoxic type I CagA-positive strains and migraine with aura. Cephalalgia, 20, 561–565.
265 Tunca, A., Türkay, C., Tekin, O., Kargili, A. & Erbayrak, M. (2004) Is Helicobacter pylori infection a risk factor for migraine? A case-control study. Acta Neurol. Belg., 104, 161–164.
266 Faraji, F., Zarinfar, N., Zanjani, A. T. & Morteza, A. (2012) The effect of Helicobacter pylori eradication on migraine: a randomized, double blind, controlled trial. Pain Physician, 15, 495–498.
267 Arzani, M. et al. (2020) Gut-brain axis and migraine headache: a comprehensive review. J. Headache Pain, 21 (1), 15.
268 Sensenig, J., Johnson, M. & Staverosky, T. (2001) Treatment of migraine with targeted nutrition focused on improved assimilation and elimination. Altern. Med. Rev., 6 (5), 488−494.
269 de Roos, N. M., Giezenaar, C. G., Rovers, J. M., Witteman, B. J., Smits, M. G. & van Hemert, S. (2015) The effects of the multispecies probiotic mixture Ecologic®Barrier on migraine: results of an open-label pilot study. Benef. Microbes, 6 (5), 641−646.
270 de Roos, N. M., van Hemert, S., Rovers, J. M. P., Smits, M. G. & Witteman, B. J. M. (2017) The effects of a multispecies probiotic on migraine and markers of intestinal permeability-results of a randomized placebo-controlled study. Eur. J. Clin. Nutr., 71 (12), 1455−1462.
271 Martami, F. et al. (2019) The effects of a multispecies probiotic supplement on inflammatory markers and episodic and chronic migraine characteristics: A randomized double-blind controlled trial. Cephalalgia, 39 (7), 841−853.
272 Maizels, M., Blumenfeld, A. & Burchette, R. (2004) A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: a randomized trial. Headache, 44 (9), 885−890.
273 Tarighat Esfanjani, A., Mahdavi, R., Ebrahimi Mameghani, M., Talebi, M., Nikniaz, Z. & Safaiyan, A. (2012) The effects of magnesium, L-carnitine, and concurrent magnesium-L-carnitine supplementation in migraine prophylaxis. Biol. Trace Elem. Res., 150 (1−3), 42−48.
274 Gaul, C., Diener, H. C., Danesch, U.; Migravent® Study Group (2015) Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: a randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain, 16, 516.
275 Guilbot, A., Bangratz, M., Ait Abdellah, S. & Lucas, C. (2017) A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement. Altern. Med., 17 (1), 433.
276 Hajihashemi, P., Askari, G., Khorvash, F., Reza Maracy, M. & Nourian, M. (2019) The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia, 39 (5), 648−654.
277 Vikelis, M. et al. (2020) Open label prospective experience of supplementation with a fixed combination of magnesium, vitamin B2, feverfew, Andrographis paniculata and coenzyme Q10 for episodic migraine prophylaxis. J. Clin. Med., 10 (1), 67.
278 Elgar, K. (2022) Vitamin D: a review of clinical use and efficacy. Nutr. Med. J., 1 (2), 52−78.
279 Pasricha, S. R., Tye-Din, J., Muckenthaler, M. U. & Swinkels, D. W. (2021) Iron deficiency. Lancet, 397 (10 270), 233−248.
280 Dib, M. (2008) Optimizing prophylactic treatment of migraine: Subtypes and patient matching. Ther. Clin. Risk Manag., 4 (5), 1061−1078. doi:10.2147/tcrm.s3983.
281 Barrow, M., Bell, L. & Bell, C. (2020) Transforming personalized nutrition practice. Nutr. Rev., 78 (12), 1046−1051. doi:10.1093/nutrit/nuaa012.
282 Martin, B. R. & Seaman, D. R. (2015) Dietary and lifestyle changes in the treatment of a 23-year-old female patient with migraine. J. Chiropr. Med., 14 (3), 205−211.