By Benjamin I. Brown
Abstract
Inflammatory bowel disease (IBD) has a complex multifactorial aetiology involving interactions between environmental factors (including diet), the microbiome, genetics and the immune system, leading to dysfunctional immune responses and chronic inflammation. Dietary factors and gut dysbiosis have emerged as important treatment targets in the management of IBD as they are involved in the initiation and perpetuation of inflammation, and subsequently disease development and progression. Specific dietary approaches and nutritional interventions have some, albeit limited, clinical evidence to suggest they can modify gene expression, have anti-inflammatory effects, induce mucosal healing, normalise intestinal microbiota, reduce disease activity and/or help maintain remission. This review uses evidence from nutritional science to propose a theoretical pragmatic model for the personalisation of nutritional therapy in patients with active or latent IBD, incorporating disease-modifying dietary recommendations and nutrient-based supplements, primarily as adjuvant therapies, with the intention to stimulate further investigation and research.
Cite as: Brown, B. (2022) Inflammatory bowel disease: towards a model for personalised nutritional therapy. Nutr. Med. J., 1 (1), 32-59.
Affiliation: B. Brown is with the Nutritional Medicine Institute, London, UK, and the British College of Nutrition and Health (BCNH), London, UK.
Corresponding author: Benjamin I. Brown (email: ben@nmi.health).
Article history: Received 25 February 2022; Peer-reviewed and received in revised form 11 March 2022; Accepted 14 March 2022. Available online 31 March 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Inflammatory bowel disease (IBD) refers to a group of chronic inflammatory autoimmune diseases, of which the most prevalent types are ulcerative colitis (UC) and Crohn’s disease (CD), that have a frequency of > 0.3% of the population in industrialised countries.1 UC primarily affects the colonic mucosa in a diffuse, continuous and superficial pattern.2 In contrast, CD can affect any section of the gastrointestinal tract, including the small and/or large intestine, the mouth, oesophagus, stomach and anus.3 Clinical symptoms of IBD include diarrhoea and/or constipation, passage of blood and/or mucus, abdominal pain and cramping, fever and signs of bowel obstruction, as well as diverse extraintestinal manifestations.4
The development of IBD involves an interaction between genetic influences, environmental factors, dysregulated immune responses and alterations of the gut microbiome.5 Importantly, genetic factors only account for a small part of disease variance, emphasising the role of gut microbial factors, alterations in intestinal immune homeostasis and environmental influences in inducing gut inflammation.6 Genetic backgrounds, life-long environmental exposures, microbial organisation and immune responses differ from person to person, and give rise to remarkably complex disease processes that are highly variable, suggesting that there are multiple disease subtypes or that each individual has a pathophysiology that is unique.7 This biological heterogeneity and complexity may help explain why traditional therapeutic interventions that target chronic inflammation have limited effectiveness and ultimately fail.8
Drug therapy of IBD centres on induction and maintenance of remission. Common drug treatments include aminosalicylates, corticosteroids, thiopurines, methotrexate and anti-tumour necrosis factor (TNF) agents.9 While drug therapy can help reduce symptoms and induce remission, 90% of people with UC and CD may experience a relapsing course of their illness.10,11 Limitations of efficacy and toxicity of traditional drug treatments might be overcome with the development of personalised therapies based on advances in understanding of disease pathophysiology.12
A better model for the management of IBD could consider the multiple antecedents, triggers and mediators that interact to produce gut immune dysregulation and uncontrolled inflammation as opportunities for personalised interventions that target an individual’s underlying pathophysiology.13 This approach has been termed ‘functional medicine’, and developed as a clinical operating model that enables healthcare providers to leverage evidence-based integrative therapies in a highly personalised way.14,15 Considerable research has provided evidence for modifiable environmental and lifestyle factors,16 microbiota-targeted therapies,17 dietary interventions,18 and herbal and nutritional medicines19 that could be individually tailored to improve acute treatment and maintenance of remission in patients with IBD.
The aim of this narrative review is to explore clinical evidence for nutritional interventions that may influence the disease course and thus be candidates for the treatment of active disease and maintenance of remission. Nutritional interventions are then organised into a pragmatic integrative model for personalised patient management that could be explored in further clinical research.
Dietary interventions
There are number of dietary risk factors for the development of IBD, many of which are typical of industrialised dietary patterns; high intakes of red meat, refined sugar, total fat and omega-6 fatty acids, and low intakes of dietary fibre, fruit and vegetables.20 Diet is a potent modifier of gene expression, gut microbial composition and mucosal immunity, all of which play a fundamental part in both risk and the progression of IBD.21 Observations from epidemiological and experimental studies have resulted in the formulation and evaluation of dietary interventions designed to halt disease progression and maintain remission in patients with IBD with clinical evidence that dietary therapy plays an important role in disease treatment (summarised in Table 3).22,23,24
Healthy diets
A traditional Mediterranean-style diet (MED-DIET) modified to reduce inflammation and exclude foods that aggravate CD was found to result in a trend towards reduction in biomarkers of inflammation [C-reactive protein (CRP) and micronuclei numbers], a change in the expression of inflammation-relevant genes, and improvement in gut bacterial diversity after 6 weeks.25 In this study, patients were provided with food items including salmon, organic avocados, sweet potato, a variety of vegetables, gluten-free bread, extra virgin olive oil, green tea, honey, and fish oil capsules. Another study assessed the multidimensional impact of a MED-DIET in patients with IBD over 6 months, and found the diet significantly reduced malnutrition-related parameters (improved body composition), liver steatosis, disease activity, and the inflammatory biomarkers CRP and faecal calprotectin.26 Similarly, a MED-DIET improved nutritional status and reduced faecal calprotectin in paediatric patients with IBD.27
A low-fat, high-fibre diet (10% of calories from fat) was compared with an improved standard American diet (35−40% of calories from fat, with increased fruit and vegetable intake) in a parallel-group, crossover study of 17 patients with UC in remission or with mild disease over 4-week dietary intervention periods. Primary outcomes were quality of life, markers of inflammation, and faecal markers of intestinal dysbiosis. Although both diets improved quality of life, the low-fat, high-fibre diet decreased markers of inflammation and reduced several biomarkers
of intestinal dysbiosis.28
A semi-vegetarian high-fibre Japanese diet that includes a gradual transition from white to brown rice, eggs, milk, miso soup, vegetables, fruits, legumes, potatoes, pickled vegetables and plain yoghurt daily, with fish once a week and meat once every 2 weeks, was found to be effective at maintaining remission in adults with CD in a 2-year prospective study. Remission was maintained at 94% in the semi-vegetarian diet group versus 33% in controls, and relapse rates at 1 year and 2 years were 0% and 8% versus 33% and 75% in controls. Furthermore, the semi-vegetarian diet was associated with normal CRP in more than half of the patients.29
Several additional reports have subsequently supported the efficacy of the semi-vegetarian diet. In a case report, the semi-vegetarian diet was found to induce remission without medication in a patient with UC that developed after a low-carbohydrate weight-loss diet.30 In another case report, the semi-vegetarian diet was used to successfully treat recent onset of UC during pregnancy.31 A study examining the effects of the semi-vegetarian diet with infliximab for inducing remission in CD (n = 44) found that 100% achieved symptomatic remission in per-protocol analyses, with significant reduction in CRP and mucosal healing achieved in 46% of cases.32 A second study of the semi-vegetarian diet and infliximab as first-line therapy for achieving remission demonstrated a high remission rate (76%; n = 17), low colectomy rate (6%), a significant decrease in CRP and erythrocyte sedimentation rate (ESR) at week 6 (9.42 mg/dl to 0.33 mg/dl, and 59 mm/hour to 17 mm/hour, respectively), and low 1-year remission rate (25%) and no additional colectomy cases.33 Most (77%) patients with mild UC or UC in remission who received hospital-based dietary education had an improvement in symptoms at 2 weeks, and relapse rates were lower than those reported for medication over a median 3.6-year follow-up.34 In a different cohort, patients with mild to severe active UC treated with the semi-vegetarian diet had a cumulative relapse rate of 14% at 1- and 27% at 5-year follow-up, considerably lower than those reported with conventional therapy (about 50% at 1 year).35
A diet low in sulphur-containing amino acids, particularly from red meat, dairy products and eggs, has been proposed to play a role in the development and progression of IBD by increasing the intestinal concentration of hydrogen sulphide and impairing butyrate synthesis.36,37,38,39 Possible adverse reactions to sulphite food preservatives E220−E228 also deserve consideration.40 Meat is an important contributor to sulphide generation by bacteria in humans.41 However, this theory is controversial as hydrogen sulphide has also demonstrated gastroprotective effects.42 Similarly, experimental studies of sulphur-containing amino acids are equivocal. Methionine may contribute to IBD pathogenesis at high dietary intakes;43 however, several studies have demonstrated gastroprotective effects of methionine and other sulphur-containing amino acids.44 Interestingly, hydrogen sulphide production may be independent of dietary sulphate and suppressed by prebiotic fibre.45
A prospective study of patients with UC in remission found that higher consumption of meat, especially red and processed meat, total protein, sulphur and sulphate were associated with disease relapse when compared with lower intakes.46 Also, a small uncontrolled pilot study assessing a low-sulphur diet [avoiding eggs, cheese, whole milk, ice-cream, mayonnaise, soya milk, mineral water and sulphite-containing drinks (wine and cordials), nuts, cruciferous vegetables, and red meat] in patients with UC suggested better maintenance of remission after 56 months versus medication with evidence of marked histological improvement, reduced number of bowel movements (from 6 to 1.5 per day) and reduced medication requirement.47 Additional evidence from semi-vegetarian diet studies discussed above suggests that a vegetarian or semi-vegetarian diet may be a feasible approach, but whether it is due to restriction of dietary sulphur or some other factor(s) is unclear.48 Dietary changes used in these studies are complex, and attributing their effects to a simple mechanism such as reduced sulphur intake may be overly simplistic and difficult to substantiate.
The IBD-AID
A multi-component, multi-functional IBD-targeted diet simplistically termed the anti-inflammatory diet (IBD-AID) was developed for the treatment of IBD, and has some evidence to suggest it is an effective regime. The aim of the diet is to induce and maintain remission, and it restricts the intake of lactose, and refined or processed carbohydrates. Foods include soluble fibre, leeks, onions and fermented foods, red meat is replaced with fish high in omega-3 fatty acid content and chicken, olive oil is used in cooking and coconut oil in baking. The diet is also personalised to account for nutritional deficiencies and food intolerances, and stages the textures of the foods to improve absorption of nutrients and minimise intact fibre depending on the symptomology of the patient. In a case series report, 60% of patients with UC and CD treated with IBD-AID had either a good or very good clinical response after reaching compliance with a large reduction in symptoms scores, and all patients could discontinue at least one of their prior IBD medications.49 In a subsequent clinical study, patients with CD were randomised to IBD-AID plus prebiotic fructooligosaccharides (FOS), FOS alone, or a placebo and control diet. The subjects were followed until either they had a flare or for up to 12 months. No subject flared in the IBD-AID group, while 31% flared in the FOS group, and 21% flared in the placebo group. There was a trend for longer survival without flare in the diet intervention group, and examination of the 16S rDNA sequencing data demonstrated significant increases in the mean abundance of the beneficial gut bacteria Roseburia after the diet intervention.50
The autoimmune protocol (AIP) diet
An AIP diet developed for IBD management was tested in patients with UC and CD. The aim of the diet was to reduce intestinal inflammation, dysbiosis and/or symptomatic food intolerance, and the regime consisted of a 6-week elimination phase (staged elimination of grains, legumes, nightshades, dairy, eggs, coffee, alcohol, nuts and seeds, refined/processed sugars, oils and food additives) followed by a 5-week maintenance phase (during which no food group reintroduction was allowed). Nutritional deficiencies in iron and vitamin D were also treated with supplementation. Clinical remission was achieved by week 6 by 11/15 (73%) of study participants, and all 11 maintained clinical remission during the maintenance phase of the study. At the end of the study period, symptom scores, quality of life and faecal calprotectin were all significantly reduced, and endoscopic improvements were noted in those who underwent follow-up endoscopy. However, CRP did not significantly change during the study, and two participants with CD with ileal strictures developed either worsening disease activity or partial small bowel obstruction.51,52 Overall, this study suggests potential benefits in UC, but the benefit and safety in CD is uncertain.
Personalised elimination diets
The use of empirical elimination re-challenge diets to identify food intolerances and construct a personalised exclusion diet has been well studied in CD.53 Typically the re-challenge phase is preceded by induction of remission with an elemental diet (a liquid nutritional formula providing essential nutrients and hypo-allergenic protein as free amino acids). In one such study, 84% of patients with active CD achieved clinical remission within 14 days of commencing an elemental diet, and were then assigned to either glucocorticoids or an elimination re-challenge diet (reintroduction of foods). Remission lengths were significantly greater in the diet group (3.8 versus 7.5 months), with 45% remaining disease-free for at least 2 years.54
Immunoglobulin G (IgG) antigens against foods have been reported to be significantly higher in patients with CD than in healthy controls, and IgG-based testing has been used with some success as a guide for an elimination diet in patients with CD.55,56 A 6-week intervention study in patients with CD found improvements in stool frequency, abdominal pain, general well-being and IFN gamma secretion of T-cells when compared with a sham diet.57 In another study, 90% of patients with CD reported symptomatic improvement, with a reduction in inflammatory biomarkers and IgG titres for the excluded foods.58 More recently, treatment of CD with an IgG-based exclusion diet resulted in significant improvements in symptoms and a reduction in faecal calprotectin in those with more severe disease.59 This approach may help maintain remission, with an IgG-based exclusion diet resulting in better control of inflammatory biomarkers and a disease relapse rate of 12.5% versus 25% in controls.60
In one study of patients with UC, an IgG-based exclusion diet for 6 months significantly reduced symptoms scores, resulted in greater reduction in extraintestinal complications, greater improvements in body composition and albumin, and improvements in quality of life when compared with a control group.61 Although most research of IgG-based exclusion diets has been in patients with CD, this report suggests a potential benefit for UC that requires further investigation.
Gluten-free diet for non-coeliac gluten sensitivity and coeliac disease
Non-coeliac gluten sensitivity (NCGS) may be more frequent in patients with IBD and could contribute to disease activity, although more research is needed to clarify the role of gluten-free diets. A majority (65.6%) of patients with IBD who have initiated a gluten-free diet independent of a diagnosis of coeliac disease report symptom improvement, and nearly 40% report fewer disease flare-ups.62 In patients with IBD, NCGS has been shown to be more frequent than in irritable bowel syndrome (IBS) and dyspeptic controls, and has been associated with more severe clinical symptoms and stricturing disease.63 Of relevance, NCGS may be related to an autoimmune phenotype with higher proportions of patients developing autoimmune disorders, positive for antinuclear antibodies, and with DQ2/DQ8 haplotypes compared with patients with IBS.64 In a case report, a patient with severe treatment-resistant UC and absence of coeliac disease achieved full clinical remission (including severe bloody diarrhoea) and improvement in laboratory data (including ESR) within 12 weeks of a gluten-free diet, with symptom exacerbation on subsequent exposure to gluten that again resolved with a gluten-free diet.65
Patients with coeliac disease have been reported to have a three−10 times higher prevalence of IBD compared with those without coeliac disease in some studies.66,67,68 Currently there are no prospective controlled studies of a gluten-free diet for patients with IBD with coeliac disease.69 However, several case reports have described important clinical improvements in patients with IBD and coeliac disease treated with a gluten-free diet and medication.70,71,72,73,74 The presence of coeliac disease should be ruled out, and in cases of confirmed coeliac disease a gluten-free diet should be initiated.
Additive-free diets
Industrial food additives such as bulking agents, colourings, emulsifiers, enzymes, flavour enhancers, preservatives, stabilisers and sweeteners have been suggested to play an important role in the development of autoimmune diseases including IBD.75 In experimental studies, several food additives have been linked to alterations in the gut microbiome, intestinal inflammation and/or the development of IBD, including the emulsifiers polysorbate-80 and carboxymethylcellulose,76,77,78 the thickener carrageenan,79 non-caloric artificial sweeteners including sucralose,80 acesulfame potassium81 and saccharin,82 the polysaccharide maltodextrin,83 the whitener and anti-caking agent titanium dioxide84, ethylenediaminetetraacetate (EDTA)85, and the widely-used (yet typically undisclosed on product labels) enzymes, microbial transglutaminases.86 Dietary exposure to such additives, in particular maltodextrin and carrageenan, has been estimated to be frequent in children with CD, although this was not related to disease severity.87
A carrageenan-free diet has been examined in patients with UC in remission. The study had two arms, a carrageenan-free diet with either placebo capsules or carrageenan capsules (200 mg/day), to tease out the effects of carrageenan exposure. Despite being a relatively small study group of 12 people, and the carrageenan capsules providing less than average daily dietary exposures, the results were striking. At the end of the study, three patients who received carrageenan-containing capsules relapsed, while none of those who received placebo-containing capsules had a relapse. And laboratory tests showed increases in the inflammatory markers interleukin (IL)-6 and faecal calprotectin in the carrageenan-exposed group, but not in the placebo group.88 Although evidence for adverse effects of carrageenan are limited, so too are safety data, and avoiding or eliminating exposure to this food additive would be advised in general. 89
A diet containing low levels of oxides of titanium, aluminium and/or silicon was found to accelerate disease remission in patients with CD treated with corticosteroids in a pilot study.90 However, a subsequent large, multi-centre, double-blind trial failed to replicate these findings.91 More investigation is needed to clarify their contribution to IBDs.92
Maltodextrin is estimated to be found in about 60% of all packaged food products, with most people consuming foods containing maltodextrin at least twice a day.93 Experimental evidence suggests that maltodextrin impairs defences against pathogenic gut bacteria, and increases the proximity of such bacteria to the epithelium.94 Importantly, analysis of mucosa-associated bacteria in people with CD showed increased prevalence of a gene essential for maltodextrin metabolism.95 However, clinical trials of maltodextrin-free diets are lacking.
In one study of 18 patients with CD, a 6-week organic diet low in ‘environmental factors’ such as fertilisers, pesticides, preservatives and food additives was found to result in significant improvements on either magnetic resonance imaging or endoscopy evaluation as well as sonography in patients with CD when compared with a control diet based on the same non-organically produced foods. However, disease activity scores were similar to the control group.96
Specific carbohydrate diet (SCD)
Originally developed for the management of coeliac disease symptoms in the 1950s,97 the SCD was popularised with the book ‘Breaking the Vicious Cycle’ in the 1980s for the management of IBD.98 The original premise for the diet was that restriction of complex carbohydrates and refined sugar from the diet would prevent malabsorption and symptom development, later the hypothesis was expanded to include the idea that malabsorbed carbohydrates could cause bacterial dysbiosis and contribute to the intestinal inflammation of IBD. Research partially supports this theory with the finding that the microbiome of patients with IBD following the SCD may become more biodiverse and characterised by higher levels of anti-inflammatory bacteria.99,100 The SCD is a modified carbohydrate diet that excludes disaccharides and most polysaccharides, but allows consumption of monosaccharides. The diet is also supplemented with homemade yogurt.
Numerous case reports suggest important clinical benefits of the SCD in paediatric patients with IBD.101,102 For example, in paediatric CD treatment with the SCD for an average of 14 months, clinical symptoms were resolved, and laboratory indices were improved or normalised, including serum albumin, CRP, haematocrit and stool calprotectin.103 Also in an adult woman with UC refractory to medication, the SCD diet improved symptoms within 3−6 months and resulted in remission, determined through colonoscopy, within 2 years.104
In the first prospective clinical study of the SCD, nine children with CD were enrolled in dietary treatment over a 12-week period, then continued the programme for up to 52 weeks. Within the first 12 weeks there was a significant clinical and mucosal improvement, with seven of the children (60%) achieving clinical remission by 12 weeks. Sustained clinical remission was seen in six of the seven patients who remained on the diet for 52 weeks.105 A subsequent 12-week study in paediatric patients with active CD compared a SCD diet with more flexible versions; a modified SCD with oats and rice (MSCD); or a whole-food diet (WF) eliminating wheat, corn, sugar, milk and food additives. All the diets were associated with 100% clinical remission and reductions in CRP and ESR, although reductions in these inflammatory biomarkers were greater in the SCD and MSCD groups, and all patients had changes in their microbiome.106
In adults with CD, an intervention trial comparing the SCD with the MED-DIET found similar clinical and inflammatory biomarker responses. Symptomatic remission at week 6 was 46% versus 43%, faecal calprotectin response was 34% versus 30%, and CRP response was 5% versus 3% for the SCD versus MED-DIET, respectively, suggesting that the MED-DIET, because it is less restrictive, may be preferred to the SCD with mild−moderate CD.107
Low-FODMAP diet
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (low-FODMAP diet) has been investigated in IBD, primarily for relief of IBS-like symptoms. In patients with IBS-like symptoms and IBD, either in remission or mild−moderate disease, a 6-week low-FODMAP diet significantly reduced IBS symptom scores and improved IBD-related quality of life.108 A 6-week low-FODMAP diet was found to reduce IBD disease activity scores and reduce faecal calprotectin in patients with mild IBD or IBD in remission.109 However, a subsequent clinical trial found improvement in IBS symptom scores but no reduction in inflammatory biomarkers.110
Genotype-guided diet
Genotype-guided personalised nutrition may have relevance in IBDs, with some evidence to suggest certain gene variations may be associated with food intolerance, and also help guide dietary interventions and nutritional supplementation.111 Regarding food intolerances, patients with CD without the GSTT1 (−/−) variant of the GST gene were found to be less likely to tolerate the brassica vegetables broccoli, cauliflower and Chinese greens in a cohort from Auckland, New Zealand.112 In the same cohort, patients carrying the G allele of FOXO3 were found to be less likely to tolerate mustard, wasabi, and raw and cooked tomatoes.113 Those with the rs1050152 variant of the organic cation transporter gene OCTN1 were found to be significantly more likely to be intolerant to mushrooms.114 Genetic lactase persistence as indicated by the T allele of rs4988235, the gene encoding for lactase-phlorizin hydrolase (lactase), had a higher risk of CD.115 As these studies were carried out in a unique cohort from New Zealand, it is important to emphasise that the same results may not always apply to other populations.116
Functional foods
In addition to more complex dietary changes, the addition of certain foods to the diet of patients with IBD has been shown to reduce disease severity. Patients with UC who added 600 g of salmon per week to their usual diet for 8 weeks had significantly reduced symptoms scores and a tendency of decreased levels of CRP.117 Patients with UC who consumed bilberries equivalent to 600 g a day of fresh berries for 9 weeks had a 90% treatment response, a 63% remission rate, a significant decrease in faecal calprotectin, and in colonic biopsies reduced pro-inflammatory and enhanced anti-inflammatory cytokines.118,119 The addition of 60 g of oat bran daily to the diet of patients with UC for 4 weeks increased stool butyrate concentration by 36%, and reduced symptoms of abdominal pain and reflux.120 Also, 50 ml of olive oil daily for 20 days decreased the inflammatory markers CRP and ESR, and improved gastrointestinal symptoms in patients with UC.121
Micronutrient deficiencies and therapy
Nutritional deficiencies are commonly associated with IBD, especially deficiencies in vitamins A, C, D, K, B3, B6, B12, folate, magnesium, calcium, iron, selenium, zinc and copper.122,123 Medications can further contribute to deficiencies, for example corticosteroid therapy reduces absorption of calcium in the intestine,124 and sulfasalazine and methotrexate increase folate requirement.125 Although micronutrient deficiencies may not be associated with overt clinical symptoms of deficiency-related disease (e.g. scurvy), they are associated with extraintestinal complications, including anaemia, bone disease, cardiovascular complications, impaired wound healing and colorectal cancer risk.126 Supplementation with micronutrients is not only important for treating deficiencies, in some cases micronutrients may help to reduce disease activity and maintain remission.127 Although several experimental studies suggest important effects of various micronutrients on colonic inflammation in IBD, human studies exploring the effects of micronutrients on disease activity are limited.128
Vitamin D deficiency is prevalent in the general population, and is 3.2 times higher in patients with IBD.129 Vitamin D treatment may help reduce disease activity and maintain remission. A randomised placebo-controlled trial of 1200 IU (30 µg) of vitamin D3 daily in patients with CD showed improvement in serum 25(OH) levels after 3 months, and more than 50% lower rate of relapse compared with placebo.130 Patients with CD treated with 1000 IU (25 µg) per day of vitamin D3 for 6 weeks had a reduction in disease activity scores and CRP.131 Supplementation with up to 5000 IU (125 µg) per day of vitamin D3 for 24 weeks raised serum 25(OH)D3, and reduced disease activity scores and improved quality of life scores.132 Notably, most patients required the maximum allotted 5000 IU of daily cholecalciferol to reach a serum 25(OH)D level of 40 ng/ml. Similarly, a clinical trial comparing a high [10 000 IU/day (250 µg)] with a low dose (1000 IU) suggested a higher dose was necessary for optimising blood levels and maintaining remission in patients with CD.133 Interestingly, clinical trials in patients with IBD suggest vitamin D treatment reduces ESR, high-sensitivity (hs)-CRP and TNF-α, increases cathelicidin (a precursor to an antimicrobial protein) gene expression, and increases gut bacterial diversity.134,135,136
Zinc has been shown to improve disease outcomes, reduce intestinal permeability and help to maintain remission. In patients with CD and UC, correction of zinc deficiency was associated with better disease outcomes, including a decrease in hospitalisations and disease-related complications compared with subjects who remained zinc deficient, over a 12-month period. Furthermore, patients with CD who normalised their zinc levels had a significant reduction in risk of subsequent surgeries.137 In patients with CD in remission, supplementation with 25 mg of zinc three times daily for 8 weeks reduced intestinal permeability and relapse rate over a 12-month follow-up period.138 Patients with active UC or ulcerative proctitis who were treated with 50 mg of zinc three times per day or placebo for 4 weeks as an adjuvant to drug therapy showed evidence of a modest improvement in clinical symptoms when compared with placebo, although this was not statistically significant.139 The doses used in these studies may be unnecessary, with doses > 40 mg daily from food and supplements associated with copper deficiency and gastrointestinal side-effects.140
Thiamine may be useful for reducing fatigue in patients with IBD. Supplementation with 600 mg (for 60 kg adults) to 1500 mg (90 kg adults) of thiamine daily for 20 days completely alleviated symptoms of fatigue in 10 out of 12 subjects with UC and CD, and the remaining two also reported a significant improvement.141 Notably there was an absence of blood thiamine deficiency, suggesting that conventional measurement may be insufficient for detecting deficiency, higher metabolic requirements or predicting treatment response. At this dose, thiamine may also be working as a pharmacological compound rather than to improve functional sufficiency.
Riboflavin has been found to have anti-inflammatory and antioxidant effects in CD. In an exploratory clinical trial, patients with CD of varying disease activity received 100 mg riboflavin for 3 weeks. Riboflavin supplementation was associated with reductions in the inflammatory biomarkers CRP, ESR, platelets and IL-2, an antioxidant effect indicated by an increase in the concentration of plasma free thiols, a reduction in clinical symptoms and an improvement in quality of life.142
Probiotics and prebiotics
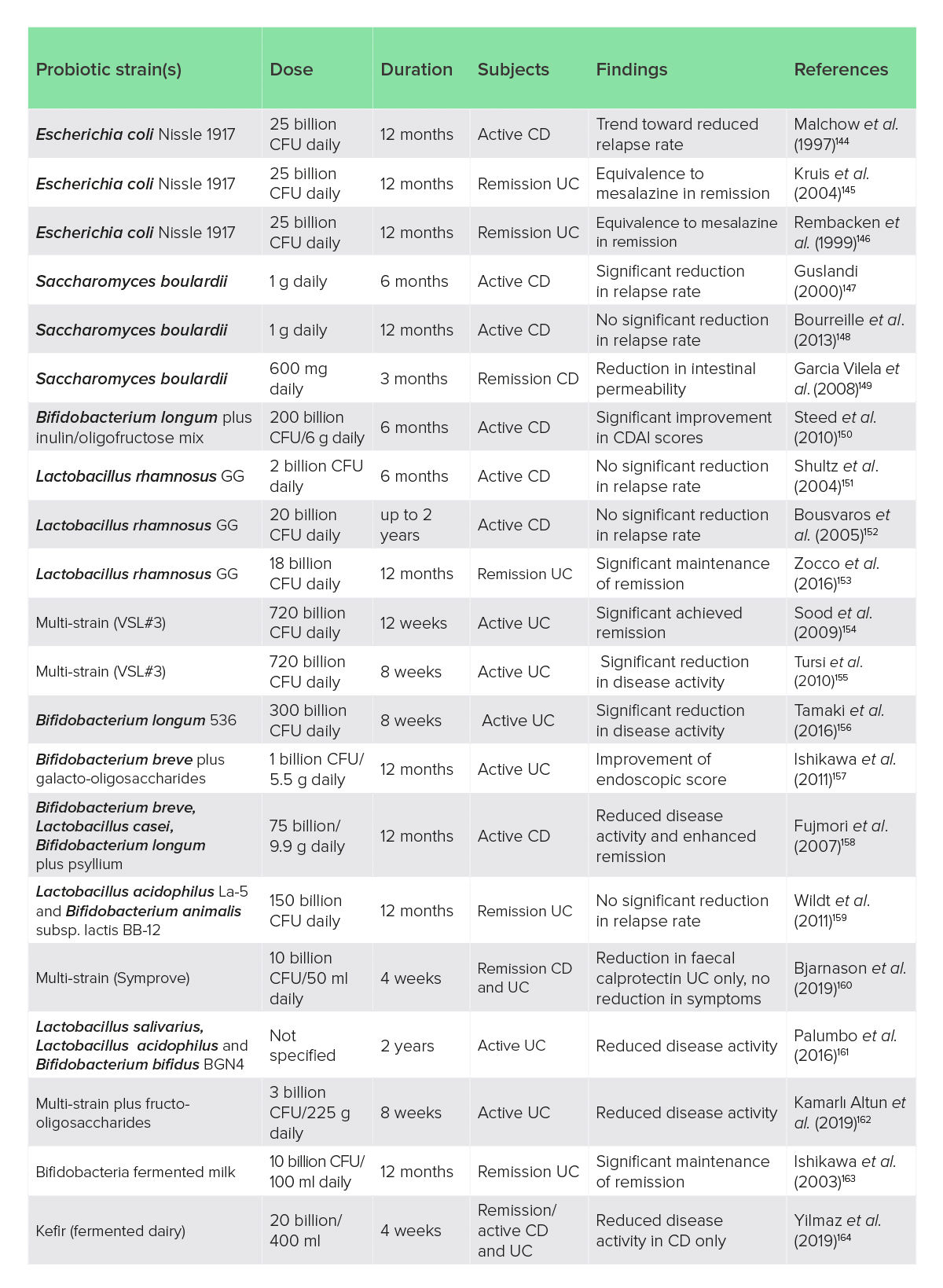
Probiotics have been the subject of considerable research in IBD but, despite a strong theoretical basis for their use, clinical studies have provided sparse and conflicting evidence, with meta-analyses and systematic reviews generally concluding that there is little evidence for efficacy in CD, modest and inconsistent data for UC, and good support for probiotics in pouchitis.143 All the clinical trials identified by the author are listed in Table 1. There is considerable heterogeneity in probiotic clinical trials related to probiotic strains, dose, duration and clinical outcomes (Table 1).
Table 1: Clinical trials of probiotics, synbiotics and fermented foods in IBD
CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CFU, colony-forming unit; UC, ulcerative colitis.
Overall, some species of probiotics have evidence of clinical efficacy in UC with less compelling data for CD, and they may be more appropriate for maintenance of remission than treatment of active disease.165 Importantly, probiotics should be used with caution with immune-suppressant drug use, long-term corticosteroid treatment, damaged intestinal mucosa or in immunocompromised patients.166 In active severe IBD with mucosal disruption, probiotics may be contraindicated as cases of bacteremia have been reported.167,168 Thus, probiotic safety is better established in less severe disease activity or IBD in remission.169
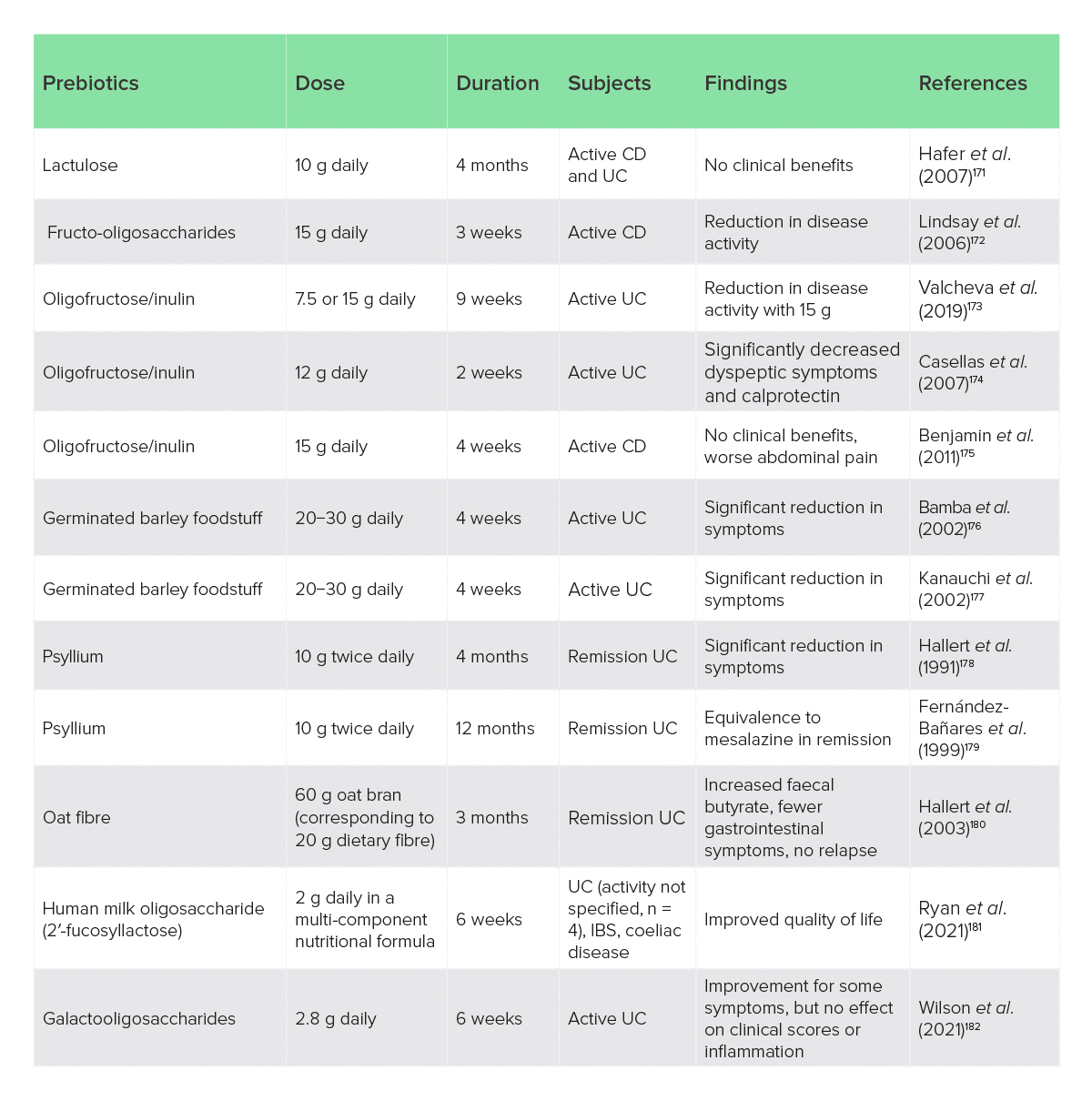
Prebiotics have considerable experimental evidence to suggest that they could modify microbial composition and lower inflammation in IBD; in contrast, clinical studies are few but generally suggest that prebiotics are well tolerated and can improve bacterial composition and reduce disease activity in UC particularly.170 The author lists all the clinical trials that they could identify in Table 2. Clinical trials of prebiotics have generally used doses of > 10 g/day for > 4 weeks (Table 2).
Table 2: Clinical trials of prebiotics and prebiotic fibres in IBD
CD, Crohn’s disease; IBS, irritable bowel syndrome; UC, ulcerative colitis.
Overall, the clinical evidence suggests some prebiotics may be useful, primarily for UC, while others may not be effective, and some could worsen symptoms. Oligofructose/inulin > 12 g daily, and psyllium (Plantago ovata) husks have more compelling evidence, with the psyllium having considerable research for gastrointestinal disorders in general to support its safety and use.183 Germinated barley foodstuff may also be useful, but the presence of gluten should be considered against the role of coeliac disease and NCGS in IBDs. Practical considerations are summarised in Table 3.
Butyrate
Butyrate is an endogenously produced short-chain fatty acid that plays a major role in the physiology of the colonic mucosa. In IBD, butyrate metabolism may be impaired, and may contribute to mucosal barrier dysfunction and inflammation.184,185.186 Enteric-coated sodium or calcium butyrate salts have been used with some success in IBD.187 In patients with mild−moderate UC, butyrate (4 g/day) plus mesalazine for 6 weeks reduced disease histology and symptoms scores better than mesalazine alone.188 Similarly, in patients with mild−moderate UC who were poorly responding to mesalazine treatment, the addition of 921 mg butyrate and 750 mg inulin resulted in a marked improvement of symptoms and in the endoscopic appearance of mucosa.189 The addition of butyrate to mesalazine in active UC for 28 days resulted in 85% experiencing a significant improvement in rectal bleeding and stool frequency compared with 55% with mesalazine alone. The butyrate group also had an increase in their faecal butyrate-producing bacteria pool, and reduced elevated baseline Bacteroides fragilis/Faecalibacterium prausnitzii ratio and lowered serum inflammatory biomarkers.190 In patients with CD with mild−moderate disease activity who took butyrate (4 g/day) for 4 weeks, 69% responded to treatment with 53% achieving remission. Further, endoscopical and histological scores significantly improved, and inflammatory biomarkers were reduced.191
N-acetylglucosamine
N-acetylglucosamine (NAG) is an amino sugar and component of epithelial cells and mucus membranes of the digestive tract.192 Experimentally, NAG has been shown to reduce intestinal inflammation and modulate the systemic immune system in autoimmunity.193,194 Metabolism of amino sugars may be impaired in patients with IBD, but NAG appears to bypass this metabolic impairment and be preferentially incorporated into the intestinal mucosa.195
Clinical studies suggest that NAG may be useful. Treatment-resistant paediatric patients with IBD were given 3−6 g per day of NAG as an adjuvant to usual therapy, in addition to rectal administration in some children. Both oral and rectal administration of NAG resulted in clear clinical and endoscopic or radiological improvement.196 In adults with IBD, treatment with 6 g NAG orally for 4 weeks resulted in an 88.1% response rate for overall clinical symptoms; a 58.8% response for abdominal pain with a 49% reduction in symptom score; a 64.7% response for diarrhoea with a 47% reduction in symptom score. There were also significant reductions in symptom scores for nausea, passage of mucus and rectal bleeding.197
Glutamine
Glutamine plays an important role in the integrity of the intestinal mucosa and regulation of the inflammatory response; however, despite its popularity as a dietary supplement for digestive health, the use of glutamine in IBD is controversial.198 One study in patients with CD in remission found that glutamine (0.5 g/kg body weight) for 2 months reduced intestinal permeability and improved morphology.199 However, other studies have not reported any benefit. Glutamine (21 g) for 4 weeks did not restore to normal the increased permeability seen in patients with CD, nor did it reduce disease activity or inflammatory biomarkers.200 In children with CD, a glutamine-enriched polymeric (exclusive enteral nutrition) diet (8 g) for 4 weeks had no advantage over a standard low-glutamine polymeric diet for symptom reduction or reducing intestinal permeability, in fact symptom control was worse with glutamine.201 Similarly, in adults with IBD, glutamine-enriched parenteral nutrition had no additional benefit.202
Creatine
The amino acid creatine is an energy precursor in the creatine kinase/phospho-creatine system, which is important for meeting the cellular energy demands of epithelial junction assembly and barrier integrity, and supplementation with creatine can restore energy synthesis and reduce disease activity in experimental models of colitis.203 In a case report, a patient recently diagnosed with mild Crohn’s ileitis started mesalamine and stopped taking a creatine supplement, after which symptoms progressively became more severe. The patient had noticed subjective improvement with creatine, they then stopped mesalamine and started creatine (1.5 g daily), after which symptoms significantly improved and mucosal healing was observed after 6 months of creatine monotherapy.204 Subsequently it has been found that patients with IBD have lower levels of creatine messenger RNA compared with control.205 The rationale for a clinical trial has been published with a suggested dose of 3−5 g twice daily.206
Phosphatidylcholine
Phosphatidylcholine is a major component of the mucus layer that forms a protective and functional barrier across the intestinal epithelium, is significantly reduced in IBD, and can be restored with phosphatidylcholine supplementation. Phosphatidylcholine comprises more than 90% of phospholipids in the mucus layer that prevents the invasion of bacteria into the intestinal epithelium from intestinal lumen, but in UC the mucus phosphatidylcholine content is reduced but as much as 70%.207 Experimentally, phosphatidylcholine depletion via diminished luminal transport leads to low mucus phosphatidylcholine, bacterial invasion of the submucosa, inflammation and IBD-like symptoms that are reversed with phosphatidylcholine administration.208
Human clinical trials of delayed-release (acid-resistant) phosphatidylcholine formulations have demonstrated important clinical benefit in active and steroid-refractory UC,209,210,211 inadequate response to mesalazine,212 and for maintenance of remission (1−4 g daily).213 A series of clinical trials of a different phosphatidylcholine formulation (differences in delayed-release technology and phosphatidylcholine %) failed to demonstrate significant clinical effect, but this has been attributed to the formulation. Earlier trials using the 30% phosphatidylcholine-containing lecithin in a delayed intestinal release formulation were found to improve clinical and endoscopic outcomes, histological activity and quality of life in patients with UC when trials of the different formulation were excluded from a meta-analysis.214 Dose−response analysis has demonstrated that optimal benefit occurs at > 1 g to 4 g daily.213
Coenzyme Q10
Coenzyme Q10 (CoQ10) or ubiquinone has shown promise in patients with mild−moderate UC. In a clinical trial, a patient with UC who received CoQ10 (200 mg daily) for 8 weeks had a significant reduction in symptom scores and blood pressure, and an improved quality of life compared with placebo.215 It was also found that CoQ10 treatment reduced inflammatory biomarkers IL-17 and nuclear factor (NF)-κB, increased serum levels of the anti-inflammatory cytokine IL-10, and increased levels of the anti-microbial peptide cathelicidin.216 Although the use of CoQ10 in UC is novel, a previous study in patients with functional gastrointestinal disorders also found an improvement in bowel movement frequency and quality of life with 150 mg of CoQ10 (as ubiquinol) daily.217 Gastroprotective effects in experimental models of colitis have also been observed.218,219
Omega-3 polyunsaturated fatty acids
The omega-3 polyunsaturated fatty acids, especially, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have undergone considerable research in IBD, but despite several clinical trials exploring potential therapeutic effects the outcomes have been mixed. Poor clinical outcomes and null effects have been observed in some studies; however, others have found modest but clinically important benefits such as improved gut histology, decreased disease activity, reduced requirement of corticosteroids and lower rates of relapse.220 A potential explanation for conflicting evidence of benefit is problems in study design, with two interesting possibilities being that dietary omega-3 to omega-6 ratio could impact treatment response, or that treatment could be personalised to people with biomarkers of inflammation.221
Supporting these possible limitations, an interesting clinical study examined the effect of 500 mg daily of EPA in patients with UC in remission, but with high elevated faecal calprotectin (≥ 150 μg/g). After 6 months, 63% of the intervention group had a 100-point reduction in faecal levels of calprotectin compared with 13.3% with placebo, and sustained clinical remission was achieved by 63.3% of patients versus 13.3% in the placebo group.222 Also, a dietary intervention that modified the omega-3 to omega-6 ratio with foods, achieving about 1700 mg/day from EPA and DHA, plus supplementation of 7 ml/day of perilla oil, providing about 3400 mg/day of alpha-linoleic acid, was able to show a significant association between higher omega-3 to omega-6 ratio and maintenance of remission in patients with UC and CD.223 Omega-3 polyunsaturated fatty acids may be a useful addition to therapy, but could benefit from personalisation to inflammatory biomarkers (e.g. elevated faecal calprotectin) and may be more effective with improvement in dietary omega-3 to omega-6 ratio. The omega-3-index (erythrocyte EPA/DHA %) corelates well with dietary EPA/DHA incorporation in gastrointestinal tissues, and may be a useful biomarker for personalising EPA/DHA therapy.224
Curcumin
Curcumin is a safe and effective therapy for improving maintenance of remission of UC when given as adjunctive therapy along with mesalamine or sulfasalazine.225 Curcumin may also help reduce acute disease activity. In an open label study, patients with active IBD were treated with 1080 mg curcumin daily for 1 month and then 1440 mg daily for the remaining 2 months. All patients with proctitis improved, and four out of five patients with CD had lowered symptom scores and ESR.226 Patients with UC who received curcumin, 1 g after breakfast and 1 g after the evening meal, plus sulfasalazine or mesalamine for 6 months had a lower rate of relapse (4.65%) compared with medication and placebo (20.51%), as well as lower symptom and endoscopic scores.227 Patients with active UC who were not responding adequately to mesalamine were given 3 g of curcumin daily for 4 weeks. Curcumin was superior to placebo and mesalamine in inducing clinical and endoscopic remission.228 Notably, a low dose of 450 mg curcumin daily was ineffective in inducing remission in mild−moderate UC.229
Boswellia
Frankincense (Boswellia serrata) has been used for centuries as a traditional medicine to treat inflammatory disorders, with some evidence to suggest it may help induce remission in IBD.230 Patients with active UC who were given 1050 mg Boswellia gum resin daily for 6 weeks achieved a better rate of remission (82%) than sulfasalazine (75%).231 Similarly in patients with chronic colitis, 900 mg Boswellia gum resin daily for 6 weeks was also found to be superior to sulfasalazine for induction of remission, with rates of 70% and 40%, respectively.232 In patients with collagenous colitis and chronic diarrhoea, monotherapy with 1200 mg Boswellia gum resin daily for 6 weeks resulted in higher clinical remission (63%) compared with placebo (26%), but had no effect on histology or quality of life.233
An enhanced bioavailability and potency extract of Boswellia (250 mg of Boswellia/lecithin complex) reduced minor symptoms, the use of drugs and medical consultation, and faecal calprotectin in patients with UC in remission.234 In patients with CD in remission, however, Boswellia was not found to be more effective for preventing disease relapse than placebo.235
Resveratrol
Resveratrol has prebiotic, immune-modulating, anti-inflammatory and antioxidant activity, with clinical evidence to suggest it can reduce clinical progression of several autoimmune diseases, including IBD.236,237 In patients with active UC, supplementation with 500 mg resveratrol for 6 weeks significantly reduced clinical symptom scores and the inflammatory biomarkers TNF-α and NF-κB when compared with placebo.238 Resveratrol also improved oxidative/antioxidative status, with a significant reduction in malondialdehyde, and an increase in superoxide dismutase and total antioxidant capacity.239
Aloe vera
Aloe vera juice has been shown to influence gastrointestinal health via promoting mucosal tissue repair, prebiotic effect, enhancing digestion, increasing absorption of nutrients and reducing inflammation.240,241,242 Treatment of 100 ml twice daily with aloe vera inner-leaf gel for 4 weeks in active UC was more effective in inducing remission (30%) than the placebo (7%). Aloe vera also significantly decreased disease activity and histological scores.243
Silymarin
Silymarin (Milk thistle extract) may be useful to help maintain remission in UC. In one study, patients who were in remission received either 140 mg silymarin or placebo once daily for 6 months along with their standard therapy. At the end of the study, 98% (n = 38) of the silymarin group maintained remission compared with 65% for placebo (n = 32). Silymarin also reduced clinical disease activity scores and improved haemoglobin and ESR when compared with placebo.244
Discussion
This review highlights a wide range of therapeutic diets and nutrient-based supplements that have potential usefulness in the integrative management of IBDs. Adjuvant nutritional therapies could be used with conventional care or could be considered as alternative treatments in cases of drug intolerance or treatment resistance. Because IBDs typically have a relapsing disease course, adjuvant nutritional therapies have promise as relatively safe interventions that could significantly contribute to disease control and improved quality of life.245 The use of nutritional therapies as alternative treatment options requires more research to establish use but, as evidenced in this review, there is clearly promise for specific interventions. Additionally, it is notable that most nutritional interventions have been studied in isolation; however, in clinical nutrition practice combinations of therapies could be personalised, may have additive effects, and may be more likely to enhance disease control and remission.
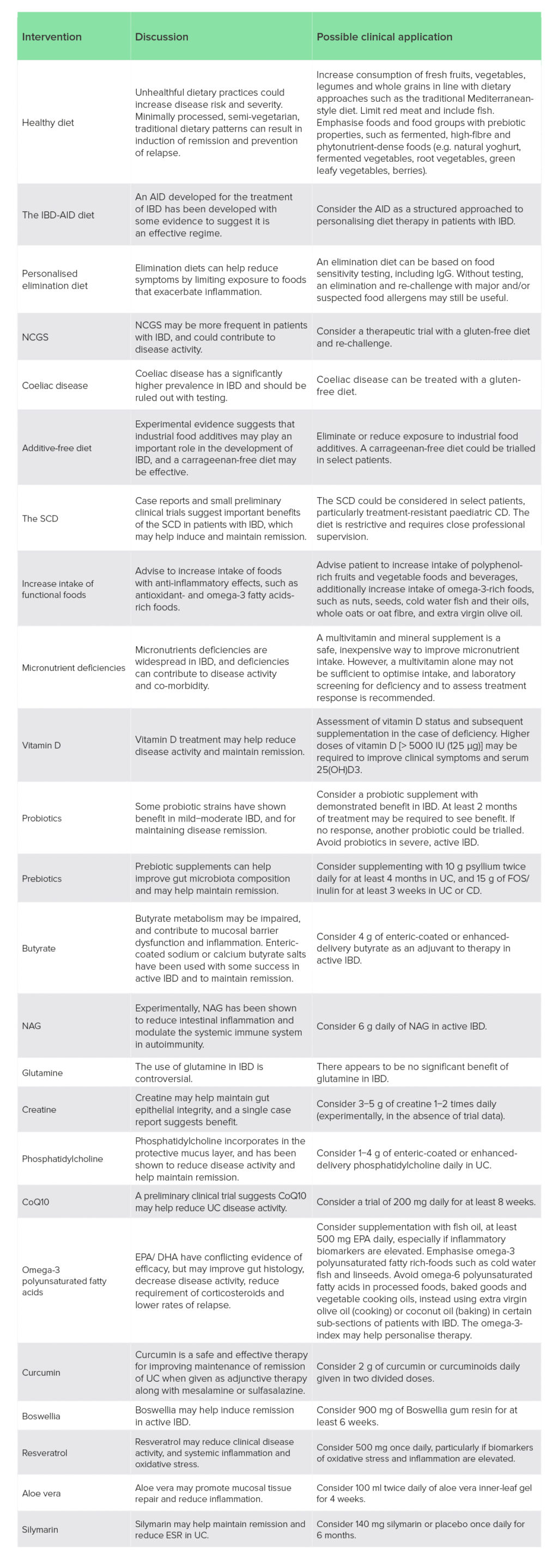
Important limitations of some nutritional therapies for IBD include a lack of suitably controlled interventions that could help inform clinical decision-making, especially in the case of dietary interventions. An example is the comparison of the SCD and MED-DIET, which both performed equally well despite the SCD being considerably more restrictive.109 Pragmatically, an easier to follow diet would be preferable, but unless such comparisons are made it may be difficult to prioritise one dietary approach over another. Mixed treatment outcomes were also frequently identified for nutritional interventions, indicating further research is needed to better understand the efficacy of candidate therapies. In the case of nutrients, many studies consisted of traditional randomised-controlled trials; however, clinical trials of nutrients would benefit from trial designs that appreciate their differences from pharmacological therapies. Unlike drugs, nutrients tend to have lower effect sizes, are influenced by background dietary intake, have dose−response curves, and benefit from personalisation.246 Nonetheless, despite these limitations, the formulation of clinical considerations based on nutritional therapies with more compelling evidence related to the therapeutic diets and nutrient-based supplements reviewed here could inform clinical application (Table 3).
Table 3: Clinical consideration of therapeutic diets and nutrient-based supplements for IBDs
AID, anti-inflammatory diet; CD, Crohn’s disease; CoQ10, coenzyme Q10; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ESR, erythrocyte sedimentation rate; FOS, fructooligosaccharides; IBD, inflammatory bowel disease; IgG, immunoglobulin G; NAG, N-acetylglucosamine; NCGS, non-coeliac gluten sensitivity; SCD, specific carbohydrate diet; UC, ulcerative colitis.
Some diets, including the AIP diet and genotype-guided diet, were excluded due to limitations in evidence. The low-FODMAP was excluded as it is for symptoms of IBS, not inducing and/or maintaining IBD remission.
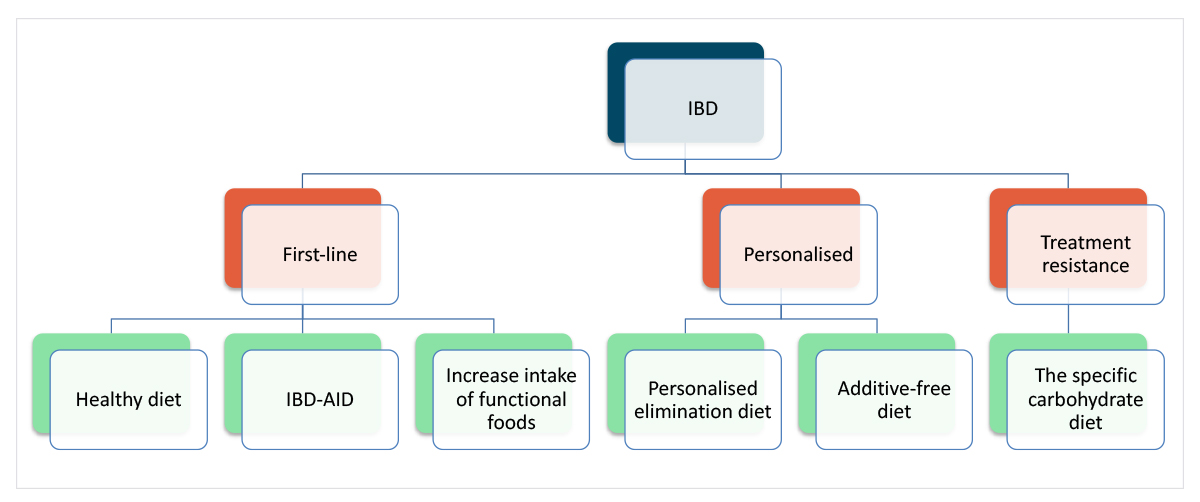
Clinically, dietary interventions could be used in combination as part of rational decision-making with lower risk, higher likelihood of benefit dietary interventions forming part of a first-line dietary approach, while more personalised elimination diets could be considered on a case-by-case basis, and more demanding and restrictive interventions being reserved for treatment resistance (Figure 1).
Figure 1: Hypothetical approach to integrating different diets for IBD.
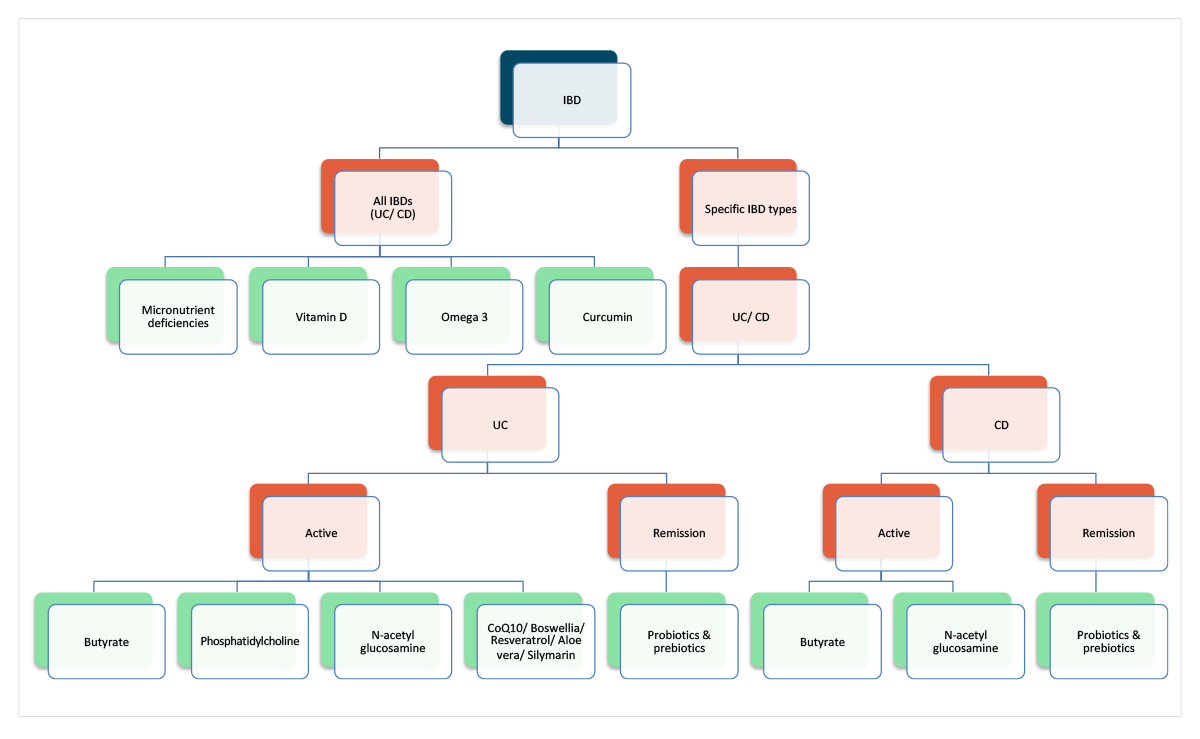
Similarly, nutrient-based supplements with supportive evidence could be approached pragmatically, prioritising those generalisable across IBDs, then personalising based on IBD subtypes and potential clinical benefit (Figure 2).
Figure 2: Hypothetical approach to integrating different nutrient-based supplements for IBD.
It must be emphasised that these are hypotheses, not guidelines, and several therapies are based on very limited research, preventing their routine use. The purpose of presenting nutritional therapies in this pragmatic, integrative model is to stimulate further investigation and research of multi-component, individualised clinical nutritional interventions for IBD.
Conclusion
Nutritional interventions clearly have great potential to reduce disease activity and maintain remission in IBDs. Diet is known to be a contributory factor to IBD development, and alterations in diet can modify the disease course. Adjuvant nutritional therapies could be used with conventional care or considered as alternative treatments in cases of drug intolerance or treatment resistance. Specific dietary approaches and nutritional interventions have some, albeit limited, clinical evidence to suggest they can modify gene expression, have anti-inflammatory effects, induce mucosal healing, normalise intestinal microbiota, reduce disease activity and/or help maintain remission. A pragmatic integrative model for the personalisation of nutritional therapy in patients with active or latent IBD, incorporating disease-modifying dietary recommendations and nutrient-based supplements holds promise, and deserves further investigation and research.
Acknowledgements
Author contributions: B. Brown carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: No funding was received for this work.
Declaration of interest: B. Brown has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA.
References
1 Ng, S. C. et al. (2017) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet, 390 (10 114), 2769−2778.
2 Ford, A. C. et al. (2013) Ulcerative colitis. BMJ, 346, f432.
3 Baumgart, D. C. & Sandborn, W. J. (2012) Crohn’s disease. Lancet, 380 (9853), 1590−1605.
4 Guillo, L. et al. (2022) Endpoints for extraintestinal manifestations in inflammatory bowel disease trials: the EXTRA consensus from the International Organization for the Study of Inflammatory Bowel Diseases. Lancet Gastroenterol. Hepatol., 7 (3), 254−261.
5 Zhang, Y. Z. & Li, Y. Y. (2014) Inflammatory bowel disease: pathogenesis. World J. Gastroenterol., 20 (1), 91−99.
6 Loddo, I. & Romano, C. (2015) Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol., 6, 551.
7 de Souza, H. S. P. & Fiocchi, C. (2018) Network medicine: a mandatory next step for inflammatory bowel disease. Inflamm. Bowel Dis., 24 (4), 671−679.
8 de Souza, H. S. P. (2017) Etiopathogenesis of inflammatory bowel disease: today and tomorrow. Curr. Opin. Gastroenterol., 33 (4), 222−229.
9 Burger, D. & Travis, S. (2011) Conventional medical management of inflammatory bowel disease. Gastroenterology, 140 (6), 1827−1837.e2.
10 Baumgart, D. C. & Sandborn, W. J. (2012) Crohn’s disease. Lancet, 380 (9853), 1590−1605.
11 Langholz, E. et al. (1994) Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology, 107, 3−11.
12 Yadav, V., Varum, F., Bravo, R., Furrer, E., Bojic, D. & Basit, A. W. (2016) Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl. Res., 176, 38−68.
13 Galland, L. & Lafferty, H. (2009) A functional medicine approach to the treatment of inflammatory bowel disease. Nat. Med. J., 1 (2), 1−7.
14 Bland, J. (2015) Functional medicine: an operating system for integrative medicine. Integr. Med. (Encinitas), 14 (5), 18−20.
15 Pizzorno, J. E. Jr (2012) Clinical decision making-a functional medicine perspective. Glob. Adv. Health Med., 1 (4), 8−13.
16 Burke, K. E., Boumitri, C. & Ananthakrishnan, A. N. (2017) Modifiable environmental factors in inflammatory bowel disease. Curr. Gastroenterol. Rep., 19 (5), 21.
17 Matijašić, M. et al. (2016) Modulating composition and metabolic activity of the gut microbiota in IBD patients. Int. J. Mol. Sci., 17 (4), 578.
18 Haskey, N. & Gibson, D. L. (2017) An examination of diet for the maintenance of remission in inflammatory bowel disease. Nutrients, 9 (3), 259.
19 Larussa, T., Imeneo, M. & Luzza, F. (2017) Potential role of nutraceutical compounds in inflammatory bowel disease. World J. Gastroenterol., 23 (14), 2483−2492.
20 Hou, J. K., Abraham, B. & El-Serag, H. (2011) Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am. J. Gastroenterol., 106 (4), 563−573.
21 Rapozo, D. C., Bernardazzi, C. & de Souza, H. S. (2017) Diet and microbiota in inflammatory bowel disease: the gut in disharmony. World J. Gastroenterol., 23 (12), 2124−2140.
22 Lee, D. et al. (2015) Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology, 148 (6), 1087−1106.
23 Kakodkar, S. & Mutlu, E. A. (2017) Diet as a therapeutic option for adult inflammatory bowel disease. Gastroenterol. Clin. North Am., 46 (4), 745−767.
24 Charlebois, A., Rosenfeld, G. & Bressler, B. (2016) The impact of dietary interventions on the symptoms of inflammatory bowel disease: a systematic review. Crit. Rev. Food Sci. Nutr., 56 (8), 1370−1378.
25 Marlow, G. et al. (2013) Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genomics, 7, 24.
26 Chicco, F. et al. Multidimensional impact of Mediterranean diet on IBD patients. Inflamm. Bowel Dis., 27 (1), 1−9.
27 Strisciuglio, C. et al. (2020) Effectiveness of Mediterranean diet’s adherence in children with inflammatory bowel diseases. Nutrients, 12 (10), 3206.
28 Fritsch, J. et al. (2021) Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol., 19 (6), 1189−1199.e30.
29 Chiba, M. et al. (2010) Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J. Gastroenterol., 16 (20), 2484−2495.
30 Chiba, M., Tsuda, S., Komatsu, M., Tozawa, H. & Takayama, Y. (2016) Onset of ulcerative colitis during a low-carbohydrate weight-loss diet and treatment with a plant-based diet: a case report. Perm. J., 20 (1), 80−84.
31 Chiba, M., Sugawara, T., Komatsu, M. & Tozawa, H. (2018) Onset of ulcerative colitis in the second trimester after emesis gravidarum: treatment with plant-based diet. Inflamm. Bowel Dis., 24 (5), e8−e9.
32 Chiba, M. et al (2017) Induction with infliximab and a plant-based diet as first-line (IPF) therapy for Crohn disease: a single-group trial. Perm. J., 21, 17-009.
33 Chiba, M. et al. (2020) High remission rate with infliximab and plant-based diet as first-line (IPF) therapy for severe ulcerative colitis: single-group trial. Perm. J., 24, 1−10.
34 Chiba, M. et al. (2018) Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: a single-group trial. Perm. J., 22, 17−167.
35 Chiba, M. et al. (2019) Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: a single-group trial. Perm. J., 23, 18−220.
36 Roediger, W. E., Duncan, A., Kapaniris, O. & Millard, S. (1993) Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology, 104 (3), 802−809.
37 Roediger, W. E., Moore, J. & Babidge, W. (1997) Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig. Dis. Sci., 42 (8), 1571−1579.
38 Owczarek, D., Rodacki, T., Domagała-Rodacka, R., Cibor, D. & Mach, T. (2016) Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol., 22 (3), 895−905.
39 Teigen, L. M., Geng, Z., Sadowsky, M. J., Vaughn, B. P., Hamilton, M. J. & Khoruts, A. (2019) Dietary factors in sulfur metabolism and pathogenesis of ulcerative colitis. Nutrients, 11 (4), 931.
40 Bold, J. (2012) Considerations for the diagnosis and management of sulphite sensitivity. Gastroenterol. Hepatol. Bed. Bench., 5 (1), 3−6.
41 Magee, E. A., Richardson, C. J., Hughes, R. & Cummings, J. H. (2000) Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr., 72 (6), 1488−1494.
42 Guo, F. F., Yu, T. C., Hong, J. & Fang, J. Y. (2016) Emerging roles of hydrogen sulfide in inflammatory and neoplastic colonic diseases. Front. Physiol., 7, 156.
43 Miousse, I. R. et al. (2017) Short-term dietary methionine supplementation affects one-carbon metabolism and DNA methylation in the mouse gut and leads to altered microbiome profiles, barrier function, gene expression and histomorphology. Genes Nutr., 12, 22.
44 Bao, X., Feng, Z., Yao, J., Li, T. & Yin, Y. (2017) Roles of dietary amino acids and their metabolites in pathogenesis of inflammatory bowel disease. Mediators Inflamm., 2017, 6869259.
45 Yao, C. K., Rotbart, A., Ou, J. Z., Kalantar-Zadeh, K., Muir, J. G. & Gibson, P. R. (2018) Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas-profiling technology. Gut Microbes, 9 (6), 510−522.
46 Jowett, S. L. et al. (2004) Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut, 53 (10), 1479−1484.
47 Roediger, W. E. (1998) Decreased sulphur amino acid intake in ulcerative colitis. Lancet, 351 (9115), 1555.
48 Tilg, H. & Kaser, A. (2004) Diet and relapsing ulcerative colitis: take off the meat? Gut, 53 (10), 1399−1401.
49 Olendzki, B. C. et al. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr. J., 13, 5.
50 Mutlu, E. A. et al. (2016) Dietary treatment of Crohn’s disease: a randomized, placebo-controlled, double-blinded clinical trial. Am. J. Gastroenterol., 150 (4, Suppl 1), S778.
51 Konijeti, G. G. et al. (2017) Efficacy of the autoimmune protocol diet for inflammatory bowel disease. Inflamm. Bowel Dis., 23 (11), 2054−2060.
52 Chandrasekaran, A. et al. (2019) An autoimmune protocol diet improves patient-reported quality of life in inflammatory bowel disease. Crohns Colitis 360, 1 (3), otz019.
53 Hunter, J. (2015) Elemental diet and the nutritional treatment of Crohn’s disease. Gastroenterol. Hepatol. Bed. Bench., 8 (1), 4−5.
54 Riordan, A. M. et al. (1993) Treatment of active Crohn’s disease by exclusion diet: East Anglian multicentre controlled trial. Lancet, 342 (8880), 1131−1134.
55 Cai, C. et al. (2014) Serological investigation of food specific immunoglobulin G antibodies in patients with inflammatory bowel diseases. PLoS One, 9 (11), e112154.
56 Kawaguchi, T. et al. (2015) Food antigen-induced immune responses in Crohn’s disease patients and experimental colitis mice. J. Gastroenterol., 50 (4), 394−405.
57 Bentz, S. et al. (2010) Clinical relevance of IgG antibodies against food antigens in Crohn’s disease: a double-blind cross-over diet intervention study. Digestion, 81 (4), 252−264.
58 Rajendran, N. & Kumar, D. (2011) Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: a pilot study. Colorectal Dis., 13 (9), 1009−1013.
59 Gunasekeera, V., Mendall, M. A., Chan, D. & Kumar, D. (2016) Treatment of Crohn’s disease with an IgG4-guided exclusion diet: a randomized controlled trial. Dig. Dis. Sci., 61 (4), 1148−1157.
60 Wang, G. et al. (2018) The utility of food antigen test in the diagnosis of Crohn’s disease and remission maintenance after exclusive enteral nutrition. Clin. Res. Hepatol. Gastroenterol., 42 (2), 145−152.
61 Jian, L. et al. (2018) Food exclusion based on IgG antibodies alleviates symptoms in ulcerative colitis: a prospective study. Inflamm. Bowel Dis., 24 (9), 1918−1925.
62 Herfarth, H. H., Martin, C. F., Sandler, R. S., Kappelman, M. D. & Long, M. D. (2014) Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis., 20 (7), 1194−1197.
63 Aziz, I., Branchi, F., Pearson, K., Priest, J. & Sanders, D. S. (2015) A study evaluating the bidirectional relationship between inflammatory bowel disease and self-reported non-celiac gluten sensitivity. Inflamm. Bowel Dis., 21 (4), 847−853.
64 Carroccio, A. et al. (2015) High proportions of people with nonceliac wheat sensitivity have autoimmune disease or antinuclear antibodies. Gastroenterology, 149 (3), 596−603.e1.
65 Rostami-Nejad, M. et al. (2017) Gluten-free diet for refractory inflammatory bowel disease; a case report. Int. J. Celiac Dis., 5 (4), 168−170.
66 Yang, A., Chen, Y., Scherl, E., Neugut, A. I., Bhagat, G. & Green, P. H. (2005) Inflammatory bowel disease in patients with celiac disease. Inflamm. Bowel Dis., 11 (6), 528−532.
67 Leeds, J. S. et al. (2007) Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand. J. Gastroenterol., 42 (10), 1214−1220.
68 Cottone, M. et al. (2003) Familial occurrence of inflammatory bowel disease in celiac disease. Inflamm. Bowel Dis., 9 (5), 321−323.
69 Weaver, K. N. & Herfarth, H. (2021) Gluten-free diet in IBD: time for a recommendation? Mol. Nutr. Food Res., 65 (5), e1901274.
70 Booth, C. (1967) A case of Crohn’s disease in a patient with treated adult coeliac disease; demonstrated at the Royal Postgraduate Medical School. Br. Med. J., 4 (5573), 222−226.
71 Euler, A. R. & Ament, M. E. (1977) Celiac sprue and Crohn’s disease: an association causing severe growth retardation. Gastroenterology, 72 (4 Pt 1), 729−731.
72 Schedel, J., Rockmann, F., Bongartz, T., Woenckhaus, M., Schölmerich, J. & Kullmann, F. (2005) Association of Crohn’s disease and latent celiac disease: a case report and review of the literature. Int. J. Colorectal Dis., 20 (4), 376−380.
73 Lail, G. et al. (2016) Coexistence of celiac and Crohn’s disease in a patient presenting with chronic diarrhea. J. Coll. Physicians Surg. Pak., 26 (6), 536−538.
74 Doya, L. J. et al., (2021) An unusual case of chronic abdominal pain: an association between Celiac disease and Crohn’s disease. Oxf. Med. Case Reports, 2021 (4), omab008.
75 Lerner, A. & Matthias, T. (2015) Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmune Rev., 14 (6), 479−489.
76 Chassaing, B. et al. (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature, 519 (7541), 92−96.
77 Swidsinski, A. et al. (2009) Bacterial overgrowth and inflammation of small intestine after carboxymethyl cellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis., 15 (3), 359–364.
78 Roberts, C. L. et al. (2010) Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut, 59, 1331–1339.
79 Martino, J. V., Van Limbergen, J. & Cahill, L. E. (2017) The role of carrageenan and carboxymethylcellulose in the development of intestinal inflammation. Front. Pediatr., 5, 96.
80 Rodriguez-Palacios, A. et al. (2018) The artificial sweetener Splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm. Bowel Dis., 24, 1005−1020.
81 Bian, X., Chi, L., Gao, B., Tu, P., Ru, H. & Lu, K. (2017) The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS One, 12 (6), e0178426.
82 Bian, X., Tu, P., Chi, L., Gao, B., Ru, H. & Lu, K. (2017) Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol., 107 (Pt B), 530−539.
83 Nickerson, K. P. et al. (2015) Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes, 6 (1), 78−78.
84 Ruiz, P. A. et al. (2017) Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut, 66 (7), 1216−1224.
85 Evstatiev, R. et al. (2021) The food additive EDTA aggravates colitis and colon carcinogenesis in mouse models. Sci. Rep., 11 (1), 5188.
86 Lerner, A., Aminov, R. & Matthias, T. (2017) Transglutaminases in dysbiosis as potential environmental drivers of autoimmunity. Front. Microbiol., 8, 66.
87 Lee, D. et al. (2018) Children with Crohn’s disease frequently consume select food additives. Dig. Dis. Sci., 63 (10), 2722−2728.
88 Bhattacharyya, S. et al. (2017) A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging, 4 (2), 181−192.
89 Borsani, B. et al. (2021) The role of carrageenan in inflammatory bowel diseases and allergic reactions: where do we stand? Nutrients, 13 (10), 3402.
90 Lomer, M. C. E., Harvey, R. S. J., Evans, S. M., Thompson, R. P. H. & Powell, J. J. Efficacy and tolerability of a low microparticle diet in a double blind, randomized, pilot study in Crohn’s disease. Eur. J. Gastroenterol. Hepatol., 13, 101–106.
91 Lomer, M. C. et al. (2005) Lack of efficacy of a reduced microparticle diet in a multi-centred trial of patients with active Crohn’s disease. Eur. J. Gastroenterol. Hepatol., 17 (3), 377−384.
92 Powell, J. J., Thoree, V. & Pele, L. C. (2007) Dietary microparticles and their impact on tolerance and immune responsiveness of the gastrointestinal tract. Br. J. Nutr., 98 (Suppl 1), S59−S63.
93 Nickerson, K. P., Chanin, R. & McDonald, C. (2015) Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes, 6 (1), 78−83.
94 Nickerson, K. P. et al. (2014) The dietary polysaccharide maltodextrin promotes Salmonella survival and mucosal colonization in mice. PLoS One, 9 (7), e101789.
95 Nickerson, K. P. & McDonald, C. (2012) Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One, 7 (12), e52132.
96 Bartel, G. et al. (2008) Ingested matter affects intestinal lesions in Crohn’s disease. Inflamm. Bowel Dis., 14 (3), 374−382.
97 Haas, S. V. & Haas, M. P. (1955) The treatment of celiac disease with the specific carbohydrate diet; report on 191 additional cases. Am. J. Gastroenterol., 23 (4), 344−360.
98 Gottschall, E. (1987) Breaking the Vicious Cycle: Intestinal Health Through Diet. Kirkton Press, Baltimore, Canada.
99 Walters, S. S. Q. A. & Rolston, M. (2014) Analysis of gut microbiome and diet modification in patients with Crohn’s disease. SOJ Microbiol. Infect. Dis., 2, 1–13.
100 Suskind, D. L. et al. (2018) Clinical and fecal microbial changes with diet therapy in active inflammatory bowel disease. J. Clin. Gastroenterol., 52 (2), 155−163.
101 Kakodkar, S., Farooqui, A. J., Mikolaitis, S. L. & Mutlu, E. A. (2015) The specific carbohydrate diet for inflammatory bowel disease: a case series. J. Acad. Nutr. Diet., 115 (8), 1226−1232.
102 Obih, C. et al. (2016) Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition, 32 (4), 418−425.
103 Suskind, D. L., Wahbeh, G., Gregory, N., Vendettuoli, H. & Christie, D. (2014) Nutritional therapy in pediatric Crohn disease: the specific carbohydrate diet. J. Pediatr. Gastroenterol. Nutr., 58 (1), 87−91.
104 Khandalavala, B. N. & Nirmalraj, M. C. (2015) Resolution of severe ulcerative colitis with the specific carbohydrate diet. Case Rep. Gastroenterol., 9 (2), 291−295.
105 Cohen, S. A. et al. (2014) Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr., 59 (4), 516−521.
106 Suskind, D. L. et al. (2020) The specific carbohydrate diet and diet modification as induction therapy for pediatric Crohn’s disease: a randomized diet controlled trial. Nutrients, 12 (12), 3749.
107 Lewis, J. D., DINE-CD Study Group et al. (2021). A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology, 161 (3), 837−852.e9.
108 Pedersen, N. et al. (2017) Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World J. Gastroenterol., 23 (18), 3356−3366.
109 Bodini, G. et al. (2019) A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition, 67−68, 110 542.
110 Cox, S. R. et al. (2020) Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology, 158 (1), 176−188.e7.
111 Laing, B. B., Lim, A. G. & Ferguson, L. R. (2019) A personalised dietary approach−a way forward to manage nutrient deficiency, effects of the Western diet, and food intolerances in inflammatory bowel disease. Nutrients, 11 (7), 1532.
112 Campbell, B. et al. (2012) Deletion of the GSTT1 genotype linked to tolerance of brassicaceae in people with Crohn’s disease in a New Zealand cohort. In: Proceedings of the Annual Scientific Meeting of the Nutrition Society of New Zealand Auckland, Auckland, New Zealand, 21–22 November 2012.
113 Marlow, G., Han, D. Y., Triggs, C. M. & Ferguson, L. R. (2015) Food intolerance: associations with the rs12212067 polymorphism of FOXO3 in Crohn’s disease patients in New Zealand. J. Nutrigenet. Nutrigenom., 8 (2), 70−80.
114 Petermann, I. et al. (2009) Mushroom intolerance: a novel diet-gene interaction in Crohn’s disease. Br. J. Nutr., 102 (4), 506−508.
115 Nolan, D. J. et al. (2010) Genetic adult lactase persistence is associated with risk of Crohn’s disease in a New Zealand population. BMC Res. Notes, 3, 339.
116 Ferguson, L. R., Hu, R., Lam, W. J., Munday, K. & Triggs, C. M. (2010) Tailoring foods to match people’s genes in New Zealand: opportunities for collaboration. J. Nutrigenet. Nutrigenom., 3 (4−6), 305−311.
117 Grimstad, T. et al. (2011) Salmon diet in patients with active ulcerative colitis reduced the simple clinical colitis activity index and increased the anti-inflammatory fatty acid index − a pilot study. Scand. J. Clin. Lab. Invest., 71 (1), 68−73.
118 Biedermann, L. et al. (2013) Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis − an open pilot study. J. Crohns Colitis, 7 (4), 271−279.
119 Roth, S. et al. (2016) Bilberry-derived anthocyanins modulate cytokine expression in the intestine of patients with ulcerative colitis. PLoS One, 11 (5), e0154817.
120 Hallert, C., Björck, I., Nyman, M., Pousette, A., Grännö, C. & Svensson, H. (2003) Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis., 9 (2), 116−121.
121 Morvaridi, M., Jafarirad, S., Seyedian, S. S., Alavinejad, P. & Cheraghian, B. (2020) The effects of extra virgin olive oil and canola oil on inflammatory markers and gastrointestinal symptoms in patients with ulcerative colitis. Eur. J. Clin. Nutr., 74 (6), 891−899.
122 Wild, G. E. et al. (2007) Nutritional modulation of the inflammatory response in inflammatory bowel disease − from the molecular to the integrative to the clinical. World J. Gastroenterol., 13 (1), 1−7.
123 Weisshof, R. & Chermesh, I. (2015) Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care, 18 (6), 576−581.
124 Chotiyarnwong, P. & McCloskey, E. V. (2020) Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol., 16 (8), 437−447.
125 Onal, I. K. (2014) Folate deficiency in Crohn’s disease. Scand. J. Gastroenterol., 49 (2), 253−254.
126 Hwang, C., Ross, V. & Mahadevan, U. (2012) Micronutrient deficiencies in inflammatory bowel disease: from A to zinc. Inflamm. Bowel Dis., 18 (10), 1961−1981.
127 Rossi, R. E., Whyand, T., Murray, C. D., Hamilton, M. I., Conte, D. & Caplin, M. E. (2016) The role of dietary supplements in inflammatory bowel disease: a systematic review. Eur. J. Gastroenterol. Hepatol., 28 (12), 1357−1364.
128 Ghishan, F. K. & Kiela, P. R. (2017) Vitamins and minerals in inflammatory bowel disease. Gastroenterol. Clin. North Am., 46 (4), 797−808.
129 Del Pinto, R., Pietropaoli, D., Chandar, A. K., Ferri, C. & Cominelli, F. (2015) Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm. Bowel Dis., 21 (11), 2708−2717.
130 Jorgensen, S. P. et al. (2010) Clinical trial: vitamin D3 treatment in Crohn’s disease − a randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther., 32, 377–383.
131 Miheller, P. et al. (2009) Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm. Bowel Dis., 15 (11), 1656−1662.
132 Yang, L. et al. (2013) Therapeutic effect of vitamin d supplementation in a pilot study of Crohn’s patients. Clin. Transl. Gastroenterol., 4, e33.
133 Narula, N., Cooray, M., Anglin, R., Muqtadir, Z., Narula, A. & Marshall, J. K. (2017) Impact of high-dose vitamin D3 supplementation in patients with Crohn’s disease in remission: a pilot randomized double-blind controlled study. Dig. Dis. Sci., 62 (2), 448−455.
134 Sharifi, A., Hosseinzadeh-Attar, M. J., Vahedi, H. & Nedjat, S. (2016) A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J. Gastroenterol., 22 (4), 316−323.
135 Dadaei, T. et al. (2015) Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterol. Hepatol. Bed. Bench., 8 (1), 49−55.
136 Schäffler, H. et al. (2018) Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis., 19 (4), 225−234.
137 Siva, S., Rubin, D. T., Gulotta, G., Wroblewski, K. & Pekow, J. (2017) Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm. Bowel Dis., 23 (1), 152−157.
138 Sturniolo, G. C. et al. (2001) Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis., 7 (2), 94−98.
139 Dronfield, M. W., Malone, J. D. & Langman, M. J. (1977) Zinc in ulcerative colitis: a therapeutic trial and report on plasma levels. Gut, 18 (1), 33−36.
140 Duncan, A., Yacoubian, C., Watson, N. & Morrison, I. (2015) The risk of copper deficiency in patients prescribed zinc supplements. J. Clin. Pathol., 68 (9), 723−725.
141 Costantini, A. & Pala, M. I. (2013) Thiamine and fatigue in inflammatory bowel diseases: an open-label pilot study. J. Altern. Complement. Med., 19 (8), 704–708.
142 von Martels, J. Z. H. et al. (2020) Riboflavin supplementation in patients with Crohn’s disease [the RISE-UP study]. J. Crohns Colitis, 14 (5), 595−607.
143 Abraham, B. P. & Quigley, E. M. M. (2017) Probiotics in inflammatory bowel disease. Gastroenterol. Clin. North Am., 46 (4), 769−782.
144 Malchow, H. A. (1997) Crohn’s disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn’s disease? J. Clin. Gastroenterol., 25, 653–658.
145 Kruis, W. et al. (2004) Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut, 53, 1617–1623.
146 Rembacken, B. J., Snelling, A. M., Hawkey, P. M., Chalmers, D. M. & Axon, A. T. (1999) Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet, 354, 635–639.
147 Guslandi, M., Mezzi, G., Sorghi, M. & Testoni, P. A. (2000) Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig. Dis. Sci., 45, 1462–1464.
148 Bourreille, A. et al. (2013) Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin. Gastroenterol. Hepatol., 11, 982–987.
149 Garcia Vilela, E. et al. (2008) Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol., 43, 842–848.
150 Steed, H. et al. (2010) Clinical trial: the microbiological and immunological effects of synbiotic consumption − a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther., 32, 872–883.
151 Schultz, M., Timmer, A., Herfarth, H. H., Sartor, R. B., Vanderhoof, J. A. & Rath, H. C.
(2004) Lactobacillus GG in inducing and maintaining remission of Crohn’s disease. BMC Gastroenterol., 4, 5.
152 Bousvaros, A. et al. (2005) A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm. Bowel Dis., 11 (9), 833−839.
153 Zocco, M. A. et al. (2006) Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther., 23 (11), 1567−1574.
154 Sood, A. et al. (2009) The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol., 7, 1202–1209.
155 Tursi, A. et al. (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol., 105, 2218–2227.
156 Tamaki, H. et al. (2016) Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc., 28 (1), 67−74.
157 Ishikawa, H. et al. (2011) Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: a randomized controlled study. Digestion, 84, 128–133.
158 Fujimori, S. et al. (2007) High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J. Gastroenterol. Hepatol., 22 (8), 1199−1204.
159 Wildt, S., Nordgaard, I., Hansen, U., Brockmann, E. & Rumessen, J. J. (2011) A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J. Crohns Colitis, 5 (2), 115−121.
160 Bjarnason, I., Sission, G. & Hayee, B. (2019) A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology, 27 (3), 465−473.
161 Palumbo, V. D. et al. (2016) The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub., 160 (3), 372−377.
162 Kamarlı Altun, H., Akal Yıldız, E. & Akın, M. (2019) Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk. J. Gastroenterol., 30 (4), 313−320.
163 Ishikawa, H., Akedo, I., Umesaki, Y., Tanaka, R., Imaoka, A. & Otani, T. (2003) Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J. Am. Coll. Nutr., 22, 56–63.
164 Yılmaz, İ., Dolar, M. E. & Özpınar, H. (2019) Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turk. J. Gastroenterol., 30 (3), 242−253.
165 Scaldaferri, F. et al. (2013) Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed. Res. Int., 2013, 435 268.
166 Sanders, M. E. et al. (2016) Probiotic use in at-risk populations. J. Am. Pharm. Assoc. (2003), 56 (6), 680−686.
167 Vahabnezhad, E., Mochon, A. B., Wozniak, L. J. & Ziring, D. A. (2013) Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J. Clin. Gastroenterol., 47 (5), 437−439.
168 Meini, S. et al. (2015) Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature. Infection, 43 (6), 777−781.
169 Sanders, M. E. et al. (2010) Safety assessment of probiotics for human use. Gut Microbes, 1 (3), 164−185.
170 Rasmussen, H. E. & Hamaker, B. R. (2017) Prebiotics and inflammatory bowel disease. Gastroenterol. Clin. North Am., 46 (4), 783−795.
171 Hafer, A., Krämer, S., Duncker, S., Krüger, M., Manns, M. P. & Bischoff, S. C. (2007) Effect of oral lactulose on clinical and immunohistochemical parameters in patients with inflammatory bowel disease: a pilot study. BMC Gastroenterol., 7, 36.
172 Lindsay, J. O. et al. (2006) Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn’s disease. Gut, 55 (3), 348−355.
173 Valcheva, R., Koleva, P., Martínez, I., Walter, J., Gänzle, M. G. & Dieleman, L. A. (2019) Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes, 10 (3), 334−357.
174 Casellas, F. et al. (2007) Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment. Pharmacol. Ther., 25 (9), 1061−1067.
175 Benjamin, J. L. et al. (2011) Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut, 60, 923–929.
176 Bamba, T., Kanauchi, O., Andoh, A. & Fujiyama, Y. (2002) A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J. Gastroenterol. Hepatol., 17 (8), 818−824.
177 Kanauchi, O. et al. (2002) Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J. Gastroenterol., 37 (Suppl 14), 67−72.
178 Hallert, C., Kaldma, M. & Petersson, B. G. (1991) Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand. J. Gastroenterol., 26 (7), 747−750.
179 Fernández-Bañares, F. et al. (1999) Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn’s Disease and Ulcerative Colitis (GETECCU). Am. J. Gastroenterol., 94 (2), 427−433.
180 Hallert, C., Björck, I., Nyman, M., Pousette, A., Grännö, C. & Svensson, H. (2003) Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis., 9 (2), 116−121.
181 Ryan, J. J., Monteagudo-Mera, A., Contractor, N. & Gibson, G. R. (2021) Impact of 2′-fucosyllactose on gut microbiota composition in adults with chronic gastrointestinal conditions: batch culture fermentation model and pilot clinical trial findings. Nutrients, 13 (3), 938.
182 Wilson, B. et al. (2021) Prebiotic galactooligosaccharide supplementation in adults with ulcerative colitis: exploring the impact on peripheral blood gene expression, gut microbiota, and clinical symptoms. Nutrients, 13 (10), 3598.
183 Singh, B. (2007) Psyllium as therapeutic and drug delivery agent. Int. J. Pharm., 334 (1−2), 1-14.
184 Segain, J. P. et al. (2000) Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut, 47, 397–403.
185 Thibault, R., Blachier, F., Darcy-Vrillon, B., de Coppet, P., Bourreille, A. & Segain, J. P. (2010) Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm. Bowel Dis., 16 (4), 684−695.
186 Cushing, K., Alvarado, D. M. & Ciorba, M. A. (2015) Butyrate and mucosal inflammation: new scientific evidence supports clinical observation. Clin. Transl. Gastroenterol., 6, e108.
187 Soldavini, J. & Kaunitz, J. D. (2013) Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Dig. Dis. Sci., 58 (10), 2756−2766.
188 Vernia, P. et al. (2000) Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: randomized, double-blind, placebo-controlled pilot study. Dig. Dis. Sci., 45 (5), 976−981.
189 Assisi, R. F., GISDI Study Group (2008) Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol. Dietol., 54 (3), 231−238.
190 Sitkin, S., Vakhitov, T. & Pokrotnieks, J. (2018) How to increase the butyrate-producing capacity of the gut microbiome: do IBD patients really need butyrate replacement and butyrogenic therapy? J. Crohns Colitis, 12 (7), 881−882.
191 Di Sabatino, A. et al. (2005) Oral butyrate for mildly to moderately active Crohn’s disease. Aliment. Pharmacol. Ther., 22, 789–794.
192 Chen, J. K., Shen, C. R. & Liu, C. L. (2010) N-acetylglucosamine: production and applications. Mar. Drugs, 8 (9), 2493−2516.
193 Grigorian, A., Araujo, L., Naidu, N. N., Place, D. J., Choudhury, B. & Demetriou, M. (2011) N-acetylglucosamine inhibits T-helper 1 (Th1)/T-helper 17 (Th17) cell responses and treats experimental autoimmune encephalomyelitis. J. Biol. Chem., 286 (46), 40 133−40 141.
194 Yomogida, S., Kojima, Y., Tsutsumi-Ishii, Y., Hua, J., Sakamoto, K. & Nagaoka, I. (2008) Glucosamine, a naturally occurring amino monosaccharide, suppresses dextran sulfate sodium-induced colitis in rats. Int. J. Mol. Med., 22 (3), 317−323.
195 Burton, A. F. & Anderson, F. H. (1983) Decreased incorporation of 14C-glucosamine relative to 3H-N-acetyl glucosamine in the intestinal mucosa of patients with inflammatory bowel disease. Am. J. Gastroenterol., 78 (1), 19−22.
196 Salvatore, S. et al. (2000) A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther., 14 (12), 1567−1579.
197 Zhu, A. et al. (2015) N-acetylglucosamine for treatment of inflammatory bowel disease a real-world pragmatic clinical trial. NMJ, 7 (4).
198 Kim, M. H. & Kim, H. (2017) The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci., 18 (5).
199 Benjamin, J. et al. (2012) Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig. Dis. Sci., 57, 1000–1012.
200 Den Hond, E., Hiele, M., Peeters, M., Ghoos, Y. & Rutgeerts, P. (1999) Effect of long-term oral glutamine supplements on small intestinal permeability in patients with Crohn’s disease. JPEN J. Parenter. Enteral Nutr., 23, 7–11.
201 Akobeng, A. K., Miller, V., Stanton, J., Elbadri, A. M. & Thomas, A. G. (2000) Double-blind randomized controlled trial of glutamine-enriched polymeric diet in the treatment of active Crohn’s disease. J. Pediatr. Gastroenterol. Nutr., 30 (1), 78−84.
202 Ockenga, J., Borchert, K., Stüber, E., Lochs, H., Manns, M. P. &Bischoff, S. C. (2005) Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur. J. Clin. Nutr., 59 (11), 1302−1309.
203 Glover, L. E. et al. (2013) Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc. Natl Acad. Sci. USA, 110 (49), 19 820−19 825.
204 Roy, A. & Lee, D. (2016) Dietary creatine as a possible novel treatment for Crohn’s ileitis. ACG Case Rep. J., 3 (4), e173.
205 Hall, C. H. et al. (2020) Creatine transporter, reduced in colon tissues from patients with inflammatory bowel diseases, regulates energy balance in intestinal epithelial cells, epithelial integrity, and barrier function. Gastroenterology, 159 (3), 984−998.e1.
206 Wallimann, T., Hall, C. H. T., Colgan, S. P. & Glover, L. E. (2021) Creatine supplementation for patients with inflammatory bowel diseases: a scientific rationale for a clinical trial. Nutrients, 13 (5), 1429.
207 Stremmel, W. et al. (2012) Mucosal protection by phosphatidylcholine. Dig. Dis., 30 (Suppl 3), 85−91.
208 Stremmel, W. et al. (2017) Genetic mouse models with intestinal-specific tight junction deletion resemble an ulcerative colitis phenotype. J. Crohns Colitis, 11 (10), 1247−1257.
209 Stremmel, W., Merle, U., Zahn, A., Autschbach, F., Hinz, U. & Ehehalt, R. (2005) Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut, 54 (7), 966−971.
210 Stremmel, W., Ehehalt, R., Autschbach, F. & Karner, M. (2007) Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Ann. Intern. Med., 147 (9), 603−610.
211 Stremmel, W., Braun, A., Hanemann, A., Ehehalt, R., Autschbach, F. & Karner, M. (2010) Delayed release phosphatidylcholine in chronic-active ulcerative colitis: a randomized, double-blinded, dose finding study. J. Clin. Gastroenterol., 44 (5), e101−e107.
212 Karner, M. et al. (2014) First multicenter study of modified release phosphatidylcholine “LT-02” in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am. J. Gastroenterol., 109 (7), 1041−1051.
213 Karner et al. (2008) T1144 results of a long-term follow-up treatment with delayed release phosphatidylcholine in chronic-active ulcerative colitis. Gastroenterology, 134 [abstract].
214 Stremmel, W., Vural, H., Evliyaoglu, O. & Weiskirchen, R. (2021) Delayed-release phosphatidylcholine is effective for treatment of ulcerative colitis: a meta-analysis. Dig. Dis., 39 (5), 508−515.
215 Farsi, F. et al. (2021) Effects of coenzyme Q10 on health-related quality of life, clinical disease activity and blood pressure in patients with mild to moderate ulcerative colitis: a randomized clinical trial. Med. J. Islam Repub. Iran, 35, 3.
216 Farsi, F. et al. (2021) A randomized controlled trial on the coloprotective effect of coenzyme Q10 on immune-inflammatory cytokines, oxidative status, antimicrobial peptides, and microRNA-146a expression in patients with mild-to-moderate ulcerative colitis. Eur. J. Nutr., 60 (6), 3397−3410.
217 Suzuki, S., Gotoda, T., Kusano, C., Ikehara, H., Miyakoshi, Y. & Fujii, K. (2019) Effect of ubiquinol intake on defecation frequency and stool form: a prospective, double-blinded, randomized control study. J. Med. Food, 22 (1), 81−86.
218 El Morsy, E. M., Kamel, R. & Ahmed, M. A. (2015) Attenuating effects of coenzyme Q10 and amlodipine in ulcerative colitis model in rats. Immunopharmacol. Immunotoxicol., 37 (3), 244−251.
219 Khodir, A. E., Atef, H., Said, E., ElKashef, H. A. & Salem, H. A. (2017) Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacology, 25 (1), 119−135.
220 Calder, P. C. (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol. Nutr. Food Res., 52 (8), 885−897.
221 Scaioli, E., Liverani, E. & Belluzzi, A. (2017) The imbalance between n-6/n-3 polyunsaturated fatty acids and inflammatory bowel disease: a comprehensive review and future therapeutic perspectives. Int. J. Mol. Sci., 18 (12), 2619.
222 Scaioli, E. et al. (2018) Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol., 16 (8), 1268−1275.
223 Uchiyama, K. et al. (2010) N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel Dis., 16 (10), 1696−1707.
224 Gurzell, E. A., Wiesinger, J. A., Morkam, C., Hemmrich, S., Harris, W. S. & Fenton, J. I. (2014) Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA+DHA content? Prostaglandins Leukot. Essent. Fatty Acids, 91 (3), 87−96.
225 Kumar, S., Ahuja, V., Sankar, M. J., Kumar, A. & Moss, A. C. (2012) Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev., 10, CD008424.
226 Holt, P. R., Katz, S. & Kirshoff, R. (2005) Curcumin therapy in inflammatory bowel disease: a pilot study. Dig. Dis. Sci., 50 (11), 2191−2193.
227 Hanai, H. et al. (2006) Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol., 4 (12), 1502−1506.
228 Lang, A. et al. (2015) Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol., 13, 1444–1449.e1.
229 Kedia, S. et al. (2017) Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: results from a randomized double blind placebo controlled trial. World J. Gastrointest. Pharmacol. Ther., 8 (2), 147−154.
230 Hamidpour, R. et al. (2013) Frankincense (r ǔ xiāng; boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complement. Med., 3 (4), 221−226.
231 Gupta, I. et al. (1997) Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur. J. Med. Res., 2, 37–43.
232 Gupta, I. et al. (2001) Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med., 67, 391–395.
233 Madisch, A. et al. (2007) Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int. J. Colorectal Dis., 22 (12), 1445−1451.
234 Pellegrini, L. et al. (2016) Managing ulcerative colitis in remission phase: usefulness of Casperome®, an innovative lecithin-based delivery system of Boswellia serrata extract. Eur. Rev. Med. Pharmacol. Sci., 20 (12), 2695−2700.
235 Holtmeier, W. et al. (2011) Randomized, placebo-controlled, double-blind trial of Boswellia serrata in maintaining remission of Crohn’s disease: good safety profile but lack of efficacy. Inflamm. Bowel Dis., 17, 573–582.
236 Chen, M. L. et al. (2016) Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio, 7 (2), e02210-15.
237 Oliveira, A. L. B. et al. (2017) Resveratrol role in autoimmune disease−a mini-review. Nutrients, 9 (12).
238 Samsami-Kor, M., Daryani, N. E., Asl, P. R. & Hekmatdoost, A. (2015) Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch. Med. Res., 46 (4), 280−285.
239 Samsamikor, M., Daryani, N. E., Asl, P. R. & Hekmatdoost, A. (2016) Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch. Med. Res., 47 (4), 304−309.
240 Blitz, J. J., Smith, J. W. & Gerard, J. R. (1963) Aloe vera gel in peptic ulcer therapy: preliminary report. J. Am. Osteopath. Assoc., 62, 731–735.
241 Bland, J. (1985) Effect of orally consumed aloe vera juice on gastrointestinal function in normal humans. Preven. Med., 14 (2).
242 Yun, J. M., Singh, S., Jialal, R., Rockwood, J., Jialal, I. & Devaraj, S. (2010) A randomized placebo-controlled crossover trial of aloe vera on bioavailability of vitamins C and B(12), blood glucose, and lipid profile in healthy human subjects. J. Diet Suppl., 7 (2), 145−153.
243 Langmead, L. et al. (2004) Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther., 19 (7), 739−747.
244 Rastegarpanah, M. et al. (2015) A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin. J. Integr. Med., 21 (12), 902−906.
245 Wang, Q., Mi, S., Yu, Z., Li, Q. & Lei, J. (2020) Opening a window on attention: adjuvant therapies for inflammatory bowel disease. Can. J. Gastroenterol. Hepatol., 2020, 7397523.
246 Heaney, R. P. (2014) Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev., 72 (1), 48−54.