By Chloe Steele
Abstract
Introduction
Bioavailability
Food Sources
General effects
Clinical uses
Safety
Conclusion
Acknowledgements
References
Abstract
Orally administered collagen in its many different forms is recognised as a highly biocompatible, safe form of supplementation, which has the potential to act on the body as an anti-inflammatory and antioxidant, and through structural remodelling and reduced lipotoxicity. The aim of this systematic review was to determine diseases where collagen has been indicated; assess safety, bioavailability and efficacy; and to provide therapeutic recommendations. It was concluded that collagen supplementation is strongly indicated for its positive therapeutic effect on pain management of osteoarthritis, balancing blood sugars in type II diabetes, wound healing, skin ageing, and post-exercise body composition and strength. Promising results were also seen for the use of collagen supplementation in osteoporosis, hypertension, rheumatoid arthritis, tendinopathy, cellulite, atopic dermatitis, sarcopenia and brittle nail syndrome. Although therapeutic recommendations were indicated in most of these diseases, owing in the large part to the use of these supplements as part of dual therapy or the uncertainty over translatability of branded products it was concluded that more studies are required to make definitive recommendations. There was a lack of clinical evidence to support the use of collagen for weight loss in obesity, gut health and in fibromyalgia.
Cite as: Steele, C. (2022) Collagen: a review of clinical use and efficacy. Nutr Med J., 1 (2): 12-36.
Affiliation: C. Steele is with the Nutritional Medicine Institute, London, England, and the Centre for Nutrition Education & Lifestyle Management (CNELM), Wokingham, England.
Article history: Received 26 July 2021; Peer-reviewed and received in revised form 30 September 2021; Accepted 8 October 2021. Available online May 31 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Collagen is the major constituent of connective and conjunctive tissues in the body, and is the most abundant protein in the human body.1 Twenty-eight different types of collagen have been found, and each is unique in its distribution, structure and function throughout the body.2 Identified using roman nomenclature, collagen type I is the main constituent of bone, skin, teeth, tendons, ligaments, vascular ligature and organs; type II is found in the cartilage; and type III is found in the skin, muscle and blood vessels.3 The remaining types have various functions throughout the body.
Orally administered collagen peptides (CPs) in supplements or food have been demonstrated to reach the bloodstream4–6 and tissues,2 indicating the potential for collagen to influence the body from within. Supplement types differ depending on the parent tissue and the extraction technique performed. Derived from gelatin, a mixture of thermal treatment and enzymatic hydrolysis results in collagen hydrolysate (CH) or specific collagen peptides (SCPs), further purification of which can result in collagen tripeptide (CTP) or octapeptide,7,8 all of which mainly contain types I and III and occasionally type II collagen.9 Unhydrolysed or undenatured collagen usually contains type II.3
Supplemental collagen is traditionally bovine, porcine or ovine sourced, but marine and synthetic vegan-derived products are now more common due to increased social and religious acceptance, and heightened awareness of recent health worries related to bovine spongiform encephalopathy and foot-and-mouth disease.10,11
The aim of this systematic review was to determine the disease areas where collagen has been indicated, and review the human clinical literature on safety, bioavailability and efficacy with a view to making specific therapy recommendations. Although topical formulations exist, this white paper focuses on orally supplemented collagen, and unless stated otherwise constitutes the research reviewed. Many forms of collagen supplement were included in this review, including CH, unhydrolysed collagen-type II (UC-II), CTP, octapeptide and SCPs.
Bioavailability
Although it is thought that orally ingested collagen is hydrolysed in the intestinal tract prior to absorption,9 native collagen and gelatin may not be efficiently absorbed compared with lower molecular weight forms that are often found in supplements,12 and there is evidence that differing forms may have differing post-prandial absorption rates, bioavailability and bioactivity.
One randomised-controlled trial (RCT) of 10 healthy males reported higher absorption rates and bioavailability of several bioactive amino acids in orally administered CH compared with non-hydrolysed collagen.13 Furthermore, CTPs containing differing amounts of the bioactive peptides glycylprolylhydroxyproline (GLY-PRO-HYP) and prolylhydroxyproline (PRO-HYP) were reported to be effectively absorbed within 1 hour in 12 human subjects, only when in the tripeptide form.14 Dose-dependent excretion of the tripeptide and dipeptide forms was also observed, indicating relative stability within the body. This is in contrast to one study that reported no differences between the bioavailability of CH and its unhydrolysed form.15 This study did acknowledge that processing methods may account for differences with other research, as differing processing techniques can influence digestibility.16
The form of CH may also dictate absorption by differing tissues within the body, indicating that unique peptide configurations may have implications for certain diseases. One animal study of CH reported absorption within 12 hours, and a higher degree of detection in cartilage if in its higher molecular weight form compared with lower molecular weight forms, indicating better bioavailability,2 which could have potential implications for cartilage-related conditions, such as osteoarthritis (OA).
Marine collagen sources have also been reported to have differing biological properties than the more commonly used land animal collagen, owing to the temperature and salinity of the water where they originate.17,18
These studies indicate that differing forms of collagen may have specific actions and efficacy within the body, and that results may not be generalisable when certain forms, brands or types of collagen are used in clinical trials.
Food sources
Vegan collagen
Collagen is a part of all animal tissues, so animal-based foods would appear to be the best sources; however, plant-based collagen products have increased on the market. Vegan collagen products are extensive in their formulations, and mainly focus around stimulating collagen production rather than supplying the original form.
In vitro studies have shown that aloe sterol, which is a plant-derived sterol resembling animal cholesterol, and andiroba oil may have the ability to stimulate collagen production; however, collagen type was not specified.19,20 Clinical research on aloe sterol focused around skin health.20,21 One in vitro study in human fibroblasts treated with aloe sterols showed increased collagen production compared with control.21 The type of collagen produced was not specified; however, it was reported that expression of two key enzymes, COL1A1 and COL3A1, which are responsible for collagen production, was increased. In the same study, a double-blind placebo-controlled trial consisted of 56 women with dry skin who were supplemented with five tablets of aloe vera gel powder per day for 8 weeks (dosage not disclosed). No differences in skin hydration were observed between the two groups; however, skin wrinkling was improved in those supplemented, and it worsened in those on placebo, but differences were not significant. A sub-analysis of subjects ≥ 40 years old showed significantly improved skin wrinkling with supplementation compared with placebo, indicating that aloe sterol may promote synthesis in those when collagen production is reduced.
Panax ginseng extract has also been shown to promote collagen synthesis in human dermal fibroblasts; however, as with aloe sterol, the type of collagen was not specified.22 In vitro studies have shown inhibited collagen type II degradation in osteoarthritic osteocytes following application of panax ginseng saponins.23 Increased collagen type I in NIH/3T3 cells, which have a potential role in wound healing,24 and diabetic fibroblasts25 has also been reported, indicating a potential for use in several different diseases. Although there is potential for panax ginseng to promote collagen I production and prevent collagen II degradation, data in humans and clinical outcomes were not evident.
Further studies in Centella asiatica, a medicinal plant known as Gotu Kola, have also indicated that collagen production may be stimulated in human skin fibroblasts when in combination with other vitamins.26 However, clinical trials in humans are lacking.
Most companies focus on boosting collagen production as previously discussed, but genetically engineering yeast to produce collagen is also possible.27 Collagen type I, type II28 and type III29 have all been produced using this method. Type I collagen was also produced in one in vitro study, which had a structure indistinguishable from animal-derived gelatin,30 but there was no evidence of its clinical use. The existence of vegan collagen on the market is very rare, but one notable form that is in production is PrimaColl™, a type 21 collagen, which is branded as the world’s first true vegan collagen.31 However, clinical trials are still in progress.
Collagen boosters make up the majority of the vegan collagen research. Although two studies were evident on supplementation of vegan collagen boosters in skin health, its application in other disease areas is lacking. As these studies are concerning collagen boosters, results from animal collagen may not be generalisable and applied to vegan collagen. No comparisons with animal-derived collagen were evident in the literature and, as products vary enormously among the industry, comparisons are difficult.
Animal-derived collagen
In recent years, bone broth as a functional food has seen a resurgence in popularity and, although it may contain nutrients that have health benefits, differences in preparation and source could result in variation in absorption rate, amino acid content, and bioavailable and bioactive peptide chains. One quantitative study of several different bone broth preparations reported lower levels of the amino acid precursors for collagen production in the commercially prepared varieties than supplemented CH powders and liquids, and home-prepared varieties and those from cafes had the highest amounts among all of the samples; reasons for this were left unexplained.32
Although bone broths may have higher amino acid content than collagen supplements, absorption rate and bioavailability are important factors to consider, and one observational study of 15 male subjects showed that although bone broth had the highest levels of collagen precursor amino acids (with the liquid collagen supplement having the lowest levels), these were more slowly absorbed than hydrolysed and non-hydrolysed collagen sources, which was attributed to the fat content of bone broth slowing digestion.15
In combination, these studies indicate that although bone broths, and in particular homemade versions, may have superior levels of collagen-promoting amino acids, the absorption rate and potentially the bioavailability of bone broth may be less than collagen supplements, especially if in the form of CH.
General effects
As previously reported in the section ‘Bioavailability’, the general effects of collagen may be specific to the type of collagen or bioactive peptide used.
Autoantigen/anti-inflammatory
Collagen has been shown in animal models to act as an autoantigen,33 with an ability to modulate inflammatory pathways. However, the exact effects of collagen on the immune system are yet to be elucidated. Reports of collagen increasing inhibitory inflammatory cytokines and decreasing proinflammatory cytokines have been found.34 UC-II is believed to activate immune cells, promoting the production of T regulatory cells, which migrate to cartilage and promote expression of anti-inflammatory cytokines interleukin (IL)-4 and IL-10.35,36 In human trials, significantly decreased inflammatory C-reactive protein but increased levels of the inflammatory molecule bradykinin following collagen administration have been reported,37 indicating a complex interaction with the immune system.
Antioxidant
The molecular weight of the collagen molecule can influence its ability to act as an electron donor and ultimately its antioxidant capacity, with enzymatic hydrolysis improving its antioxidant capacity.38 CH also contains hydrophobic amino acids, such as aromatic amino acids and histidine, which are known to have antioxidant properties; however, the exact mechanisms are still unknown.39
Structural remodelling
The main action of collagen supplementation is its ability to promote endogenous collagen production,40 which is the main structural protein in the body. Clinical trials have reported that collagen can influence structural remodelling through promotion of the cartilage matrix,41 and an increase in bone remodelling through stimulation of amino-terminal propeptide of type I collagen (P1NP), whilst maintaining the bone degradation protein C-telopeptide of type I collagen (CTX 1).42,43
In addition, the presence of two peptides PRO-HYP and hydroxyprolyl-glycine (HYP-GLY) in collagen has been shown in in vitro studies to enhance cell proliferation and enhance the production of hyaluronic acid in dermal fibroblasts.44,45
Lipid lowering
Lipotoxicity can result from excess fat accumulation, and can impair numerous metabolic pathways within multiple organ systems. The most prominent of these are the insulin signalling pathways such as the novel protein kinase C pathway and the JNK-1 in adipose and muscular tissues, which if impeded can result in insulin resistance.46 Animal models have reported reduced total cholesterol, low-density lipoprotein cholesterol (LDL-c) and triglycerides following administration of hydrolysed marine collagen peptides (HMCPs),47 which may have an impact on several areas of the body.
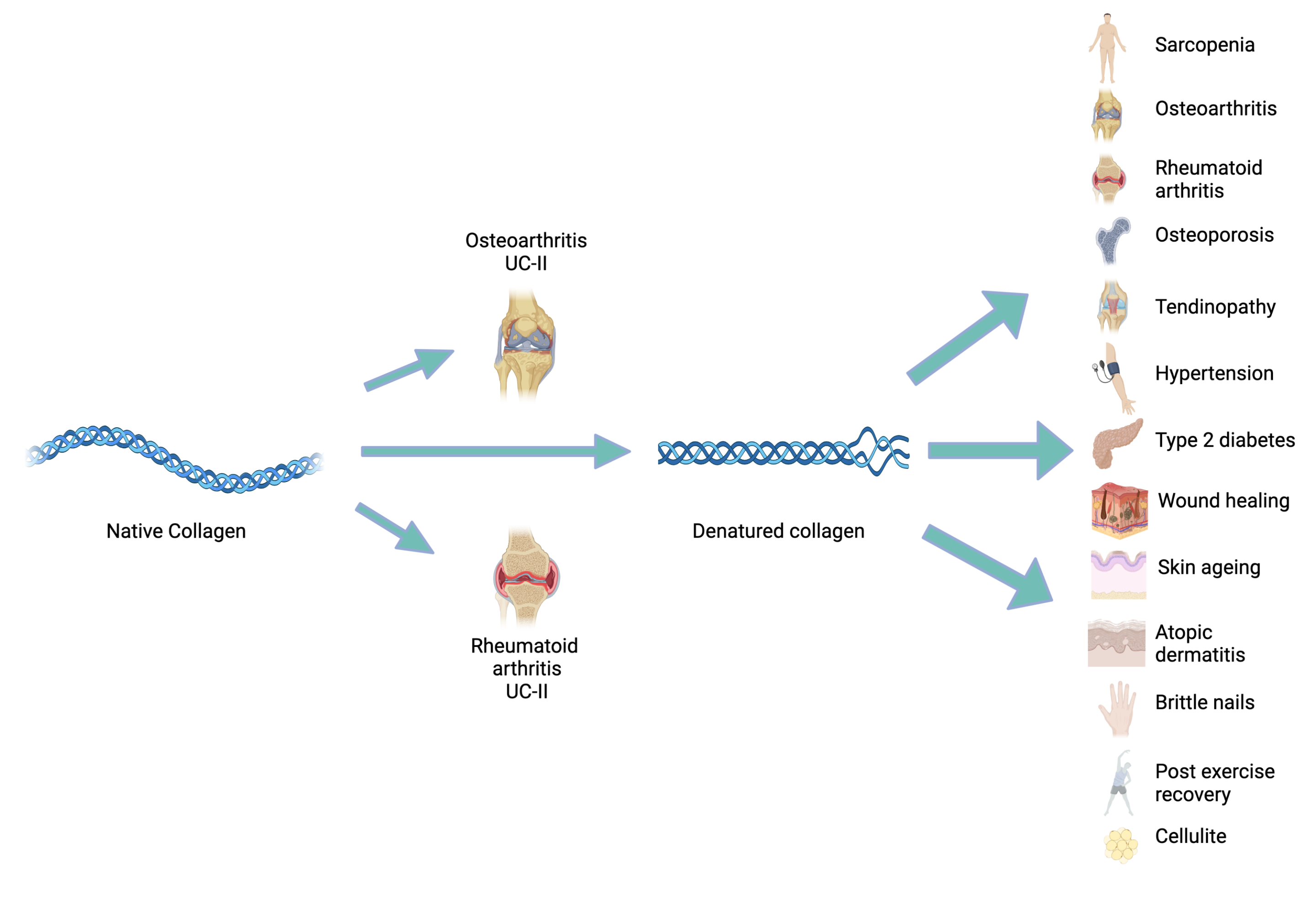
Figure 1: Areas where collagen supplementation is clinically indicated.
Figure 1: Native collagen in the form of undenatured collagen type-II (UC-II) has been indicated for use in both osteoarthritis (OA) and rheumatoid arthritis (RA). When chemically denatured, to produce collagen hydrolysate (CH), specific collagen peptides (SCPs), bioactive collagen peptides (BCPs) or hydrolysed marine collagen peptides (HMCPs), its use has been indicated in several disease areas.
Clinical uses (Figure 1)
Osteoarthritis (OA)
Osteoarthritis is the most common joint disease globally48 and, in the absence of disease-altering medications, pain management is the only option for individuals with this disease.49 As a result, increasing interest has developed for the potential of nutraceuticals to help manage symptoms.
Osteoarthritis is not an autoimmune disease, so mechanisms for the action of collagen in this disease have been of interest, and three have been highlighted from in vitro, animal studies and clinical trials. Firstly, the peptides that collagen contains may stimulate the building blocks for the cartilage matrix,41 secondly it may influence bone metabolism,50,51 and finally it may work via the vascular system to improve symptoms.52–54
Inhibition of angiotensin I-converting enzyme (ACE) and regulation of nitric oxide (NO) and soluble intercellular adhesion molecule-I (ICAM-I) have been shown in animal models following treatment with chicken CH.52 OA is often associated with venous insufficiency55 and low-grade inflammation,56 which can affect the vascular system.
Twelve studies were found on the use of collagen in OA, and the majority of these concentrated on CH, although a small amount of research has been conducted using UC-II. Diagnosis and the criteria measured in the majority of the literature appear to centre around self-reported symptom severity based on the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Visual Analogue Scale (VAS), which are both universally recognised.57
Mixed outcomes were reported among the systematic reviews and meta-analyses. One of the meta-analyses that included five studies on collagen in OA reported improvements to pain in the short, medium and long term; however, physical function was not improved compared with placebo with either CH or UC-II.58 A second meta-analysis of five RCTs supported this finding in only one of the pain parameters measured, concluding that OA is effective in improving symptoms of pain.59 Two of the studies that were included had a treatment period of less than 14 weeks, which may be insufficient to observe symptom relief and could account for an improvement in only one of the assessments for pain. A third systematic review of 48 human and animal studies concluded that collagen and its derivatives had beneficial effects to patients suffering from OA in pain and physical function; however, no meta-analysis was performed.60
These trials supported the use of collagen and its derivatives for the relief of OA symptoms; however, one meta-analysis of eight studies concluded that there was insufficient evidence to support the use of collagen in OA.61 However, it should be noted that gelatin was included in this analysis, which is the unhydrolysed form of collagen, and it is thought that dietary collagen that has not been hydrolysed is poorly absorbed,62 as previously mentioned.
Supplemental doses of CH used in the literature were mainly 10 g/day, and one RCT of 250 patients with knee OA reported significantly decreased pain and improved functioning following supplementation of 10 g CH daily over 6 months compared with placebo, results that were seen at all stages of OA.63
Contrary to the trial above, disease severity may dictate outcomes. One pilot RCT of 30 individuals with mild knee OA over 24 weeks reported no symptom relief with 10 g/day CH, but magnetic resonance imaging (MRI) changes were detected,64 indicating that although symptoms may not have been relieved, internal improvements were affected. A second RCT of 389 patients supplemented with 10 g/day CH for 24 weeks reported no significant differences with placebo on measures of pain and physical function.2 However, a subset of patients with extreme disease did report significantly improved symptoms, indicating that differences between mild and extreme disease may influence success of supplementation. Interestingly, in patients with extreme disease, symptom relief perpetuated after supplementation had ceased, suggesting long-term benefits for symptom relief.
Collagen derivatives are not the only nutraceuticals used in the relief of OA symptoms, and one RCT showed significantly reduced symptom relief with 10 g/day CH compared with glucosamine sulphate (GS) in a 13-week trial of 100 patients with knee OA, effects that were seen as early as 2 weeks after therapy commenced.65 These results were supported in another RCT using 40 mg/day UC-II versus GS66 and 10 mg/day UC-II versus GS plus chondroitin,67 suggesting that collagen derivatives may have benefits above the most commonly used nutraceuticals for OA.68
Collagen formulations have also been investigated in relation to OA symptoms. One RCT that looked at a combination of CH (type II), chondroitin sulphate and hyaluronic acid (BioCell® collagen) containing 300 mg CH/day for 70 days was shown to improve VAS score but not WOMAC; short therapy duration was implicated in this discrepancy,62 but this may also be related to suboptimal dosing based on the use of 10 mg/day in previous studies.
Several studies were found that focused on individuals without OA but relating to joint health. The research supported supplemental doses of CH of 1.2 g/day for improvements in joint pain but only if taken for at least 6 months,69–71 as one study showed no symptom improvement over 12 weeks, even if 10 g/day was taken.72
There is a large amount of quality literature supporting the use of 5−10 mg/day CH for the long-term relief of OA symptoms especially in those with extreme disease, and these benefits may be superior to the more commonly used GS and chondroitin. Positive results for the use of 40−300 mg/day UC-II in symptom relief of OA was also evident. Research in the use of BioCell® may only be translatable when using this specific brand of supplement.
Rheumatoid arthritis (RA)
Rheumatoid arthritis is considered to be an autoimmune disorder characterised by infiltration of the joints by immune cells, attacking the collagen and resulting in chronic pain and inflammation.33 Collagen has been shown in animal models to act as an autoantigen, supressing arthritis.33
When compared with standard therapy (methotrexate), collagen may not be as effective, but has less adverse events associated with it. One double-blind RCT of 211 patients with RA supplemented with 0.1 mg/day UC-II for 24 weeks reported that although improvements to pain and function were observed from the start of the trial, these were less than with standard therapy.73 Regardless, given the improvements from the start of the trial, it was concluded that UC-II is still effective for the treatment of RA. These results, dosages and patient types were replicated in a second study of 503 patients.74
Stopping methotrexate supplementation in favour of collagen may result in reversal of symptom benefits. Among 92 patients with RA in a double-blind RCT, those who were first given methotrexate and then switched to UC-II 0.5 mg/day resulted in worsening of swollen joints. Those who remained on standard therapy reported no change in disease activity, indicating an inability to sustain the results of standard therapy.75 Unfortunately no comparisons were made with a placebo group.
The previous trials indicate that UC-II supplementation may not be as effective as standard therapy; however, it could be used to enhance standard therapy. In one early RCT of 60 patients with severe RA, UC-II was reported to improve symptoms of RA after 3 months when compared with placebo, and four of the collagen participants reported complete resolution of disease.76 Sixty-four percent of the collagen-treated patients were on standard therapies, usually methotrexate, and further improvement occurred with collagen, indicating an additive effect of collagen, which was not investigated in the previously reviewed methotrexate studies. Unfortunately, collagen dosages were not stated
in the trial.
When looking at differing doses of UC-II, one double-blind placebo-controlled RCT of 274 patients reported improvements in one measure of RA symptoms, the Paulus criteria, which is a measure of joint soreness, swelling and morning stiffness, with the lowest dose of 20 µg but not at the higher doses of 100, 500 and 2500 µg.34 However, other measures of disease improvement were not observed. The dosage used in this trial was very different to other trials.
One observational study looked at the use of low-dose UC-II in juvenile RA; however, the sample size of 13 individuals was too low to show any definitive treatment effect, but six patients who were given 60 µg UC-II and six patients who were given 540 µg reported improvements in disease symptoms. However, it was concluded that UC-II efficacy in juvenile RA may be difficult to prove.
Studies are mixed for the use of collagen in RA, maybe owing to studies focusing on higher dosages, as it has been reported that the immunosuppressive action of autoantigens is usually observed at lower doses.77 RA did improve in certain patients, and efforts to identify individuals who may see improvements in disease are needed. Benefits were apparent for the management of RA at 20−100 µg/day, and dual therapy of UC-II and methotrexate may improve standard therapy outcomes but should not replace it in adults. If side-effects from methotrexate do preclude treatment, UC-II may be a good alternative, as it was still shown to be effective.
Osteoporosis
The complex aetiology of osteoporosis involves both modifiable and non-modifiable risk factors, such as malnutrition, lack of exercise, age and gender.42 Current non-pharmacological strategies to maintain bone health involve supplementation with vitamin D and calcium.78 However, as type I collagen is a component of bone,79,80 supplementation may have some benefit.
In combination with vitamin D and calcium, 5 g/day CH was shown in an RCT of post-menopausal women to slow decreases in body bone mass density (BMD) over 12 months.81 In a second RCT where 5 g/day SCPs was combined with calcium and vitamin D in post-menopausal women over 3 months, enhanced benefits in bone formation compared with calcium and vitamin D dual supplementation were observed; however, bone degradation remained unchanged.43 Furthermore, in one RCT with 5 g SCPs/day, BMD was increased compared with decreased levels with placebo, and this was attributable to increased bone stimulation markers in the blood; however, bone degradation markers remained unchanged.42
In combination, these trials indicate the possible complementary effects of collagen, vitamin D and calcium, with collagen acting on bone formation82 and not degradation. Increased bone formation may have implications for osteoporosis reversal; however, this needs to be better researched in the long term and in relation to bone resorption.
In contrast, an RCT that focused on supplementation of 10 g/day CH in 82 post-menopausal women did not show any differences in bone formation after 24 weeks compared with placebo, but it should be noted that this study looked at differing measures of bone formation and degradation compared with the other studies reviewed, and collagen may be specific to certain biomarkers.
There were mixed results among the few studies on the use of collagen in osteoporosis, possibly due to different measures of bone turnover throughout the studies. Research for the use of 5 g CH and SCPs in osteoporosis is promising; however, as most of the research is in combination with vitamin D and calcium, recommendations should involve dual supplementation. Also, CHs may have the ability to bind calcium ions, which improves bioavailability,83,84 and could also account for the synergistic actions observed.
Tendinopathy
Treatments for tendinopathy focus on exercise programmes but results are inconsistent, with a large majority of sufferers failing to see results.85 As collagen-derived peptides have been reported to modulate collagen synthesis41 and joint-related pain,70,71 the use of collagen in tendinopathy is promising; however, it appears that research in this area is limited.
One pilot crossover RCT on 20 individuals suffering from Achilles tendinopathy reported that 5 g/day CH for 6 months in the branded supplement TENDOFORTE® in combination with exercise improved self-reported assessments of pain and improved tendon health as indicated by ultrasound. However, tendon health also improved in the placebo group, making it difficult to determine whether improvements were because of CH or due to exercise, which is
the current recognised strategy for treatment of tendinopathy.
The results above are promising for the use of 5 g CH in tendinopathy; however, it cannot be definitively concluded that collagen was solely responsible for the effects observed. Based on the current research, the recommendation of 5 g/day CH in combination with strength exercises may improve symptoms of tendinopathy.
Obesity-associated weight loss
The aetiology of obesity is complex, involving the interrelation of many physiological, psychological, genetic, sociological and economic factors contributing to development.86 The management of weight loss involves lifestyle management, in some cases pharmacotherapy and in extreme cases bariatric surgery.87 Diet-induced weight loss has been associated with a reduction in bone health and BMD,88 which is where collagen supplementation may be of interest; however, no studies were found investigating this. CPs in animal studies have demonstrated the ability to regulate lipid metabolism and adipokines involved in energy intake and weight maintenance.89
High-protein diets have been reported to aid and help maintain weight loss in obese individuals,90 and collagen supplements could be a nutritional strategy to increase protein intake. One RCT of 2000 mg/day skate collagen peptides in 90 overweight adults for 12 weeks reported that CPs improved body composition compared with placebo, with changes observed from 6 weeks of supplementation.91 It should be noted that the study size was relatively small, and although the authors state that exercise levels and dietary intake were assessed at baseline, these were not stated at the end of the study and so effects may be influenced by this.
In an 8-week study of 37 overweight women, 38 g/day CH as a protein supplement compared with 40 g/day whey protein reported an increased body mass index. Furthermore, body composition in the whey protein group was significantly improved compared with CH. It was concluded that observed increased branched-chain amino acids that are involved in satiety may have been responsible for the effects observed in the whey protein group, and that collagen may not be an effective protein weight loss aid for overweight women.92
It cannot be ruled out that exercise levels and dietary intake were responsible for the positive results shown in the research above. Differences in the types of collagens used, the treatment cohort and the duration of study may also be responsible for the controversial results reported in the use of collagen for weight loss in overweight individuals. The main aim of the above weight loss diets was to use collagen as a high source of protein, yet collagen and its derivatives may be of low nutritional value due to deficiency or absence of some essential amino acids.93 As a result, it is concluded that there is a lack of research for the use of collagen for weight loss.
Hypertension
Current reports suggest that 1.13 billion people suffer from hypertension worldwide,94 indicating a need for better prevention and management strategies. Animal studies and clinical trials have indicated that CH may activate endothelial progenitor cells, modulate the renin-angiotensin aldosterone system95 and promote vascular relaxation,52 resulting in improved blood pressure.
One observational study showed that supplementation with 5.2 g of chicken collagen octapeptides in 15 individuals with mild hypertension resulted in significantly reduced systolic (SBP) and diastolic blood pressure (DBP) after 2 and 4 weeks.95 In support of this, an RCT on collagen in osteoporotic post-menopausal women showed that both SBP and DBP were decreased following 5 g SCPs for 12 months; however, this was not the primary outcome measure and this cohort of patients was not diagnosed with hypertension at the start of the trial.42 Furthermore, long treatment duration may be responsible for the resultant effects.
In contrast, an RCT of 58 people with mild hypertension receiving 2.9 g CH reported significantly decreased SBP in comparison to placebo, but no significant differences in DBP after 18 weeks.96 Furthermore, an RCT of 13 g/day HMCPs for 3 months in 150 adults diagnosed with primary hypertension reported significantly reduced DBP; however, no differences in SBP were observed between the groups.97 NO levels were decreased, suggesting that NO-regulated vasodilation was not responsible for this change.
Research is promising for the use of collagen for hypertension, but robust long-term RCTs with benefits to SBP and DBP are lacking. Treatment durations and type of collagens used could account for conflicting results among the studies. The research reviewed indicates the use of at least 5 g/day CH and 13 g/day HMCPs for hypertension; however, more studies are needed in larger subject groups and those looking at dose−response in various stages of hypertension to make definitive recommendations.
Type 2 diabetes (T2D)
Type 2 diabetes is being described in the literature as a disease of epidemic proportions,98 with an estimated 1.5 million deaths worldwide in 2019 directly due to diabetes.99 Previous animal studies have shown promising results in the use of collagen for the reduction of hyperlipidaemia47 and, as lipotoxicity in the pancreatic and muscle cells may inhibit insulin receptor signalling contributing to insulin resistance,100 collagen may improve glucose metabolism through its actions on a key pathophysiological process of T2D.
In a prospective RCT of 100 patients with T2D and hypertension, 13 g/day HMCPs for 3 months resulted in improved blood sugar control compared with placebo, and these results were seen as soon as 1.5 months into the study,101 indicating that disease progression was not simply slowed, but improved. Insulin sensitivity was increased with HMCPs; however, this remained unchanged in the patient control group. Treatment improved the lipid profile of the participants and, although causal relationships were not investigated, it could be speculated that lipid regulation improved insulin sensitivity resulting in improved blood glucose control.
A second 3-month RCT of HMCPs by the same authors of the previous study looked at 13 g HMCPs in 100 patients with non-hypertensive T2D and, in support of the findings above, blood glucose control and insulin sensitivity were improved compared with the placebo group.37 In this study, the modulation of inflammation-related metabolic regulators was observed in HMCPs compared with control, and may contribute to the regulation of blood sugar.
The research is limited for the use of collagen in T2D; however, the literature that does exist is positive and the quality is high. What is apparent is that 13 g HMCPs may be of benefit to hyperglycaemia for a duration of at least 6 weeks.
Fibromyalgia
Fibromyalgia is characterised by widespread musculoskeletal pain, resulting from neuroendocrine dysfunction.102
One study was found on the use of collagen in fibromyalgia; however, this observational study over a 90-day period with 20 fibromyalgia sufferers failed to state dosages and collagen type, and although it concluded improvements to symptoms, there were no statistical analyses and the study was not blinded.103 Therefore, it can be concluded that there is a lack of research for the use of collagen for the relief of fibromyalgia symptoms.
Wound healing
Although wounds caused by pressure ulcers or burns have very different aetiologies, both appear to include nutritional support and dietary supplements as part of their management plans.9,104 As collagen is a major component of skin and studies involving collagen-based dressings in diabetes-related ulcers have shown efficacy on wound healing,105 oral supplementation may be of interest.
One double-blind RCT of 16 weeks in 120 individuals suffering from pressure ulcers reported that 10 g/day CH, rich in PRO-HYP and HYP-GLY, showed improved wound healing, measures of pain and wound area compared with placebo after 16 weeks. Interestingly, this study had a third group allocated a PRO-HYP, HYP-GLY-deficient CH supplement and although no differences were found between the two CH groups, this arm did report improvements in wound healing compared with placebo,9 indicating that improvements were regardless of amino acid content.
This was supported in a second RCT where 89 patients with pressure ulcers were given 15 g/day CH and showed improved ulcer healing compared with placebo. The cumulative improvement in ulcer healing was 96% greater in the treatment group than in the control.106
Different wound types were also investigated, and one double-blind RCT in 31 men with burns covering 20−30% of their body reported improved wound healing 3.7 times higher than placebo when taking 36 g/day CH and, although hospitalisation duration was not significantly lower with CH, it was deemed clinically significant. Results were attributed to better improvements in serum albumin levels observed in the CH cohort.104
Although not extensive, the research in the use of CH for wound healing is of high quality, and in pressure ulcers supports the use of 10−15 g CH/day for at least 16 weeks if taking the lower dose and 8 weeks if taking the higher dose. In burns, the research is also of very good quality, and supports the use of 36 g/day CH to improve wound healing and to clinically improve duration of hospital stay.
Skin ageing
The skin is the body’s largest organ, with its main role being protection. It is comprised of two layers; the dermis is the deepest and collagen is its main constituent. Damage to the skin can be caused in a number of ways: UV radiation, nutrition, pollution and cigarette smoke can all result in collagen damage and subsequent extrinsic ageing.107 Intrinsic changes involve an age-related decrease of collagen production by approximately 1% per year.108 In tandem, this can result in the breakdown of the bonds holding the dermis to the epidermis resulting in wrinkling and decreased elasticity.109
Studies have shown that creams and lotions may be unable to reach the dermis to causally affect collagen production.110,111 The aim of oral collagen supplementation is to reach the dermis from within, to restore collagen synthesis and influence the skin ageing process, and may be an alternative or additive to topical treatments. PRO-HYP derived from dietary collagen may enhance the growth of fibroblasts and promote synthesis of hyaluronic acid.110
Fourteen studies were found on the use of collagen and its derivatives for skin ageing during the searches, comprising one systematic review and meta-analysis, ten RCT’s and three observational studies. Improvements were seen in measures of skin ageing across all dosages in one systematic review.112 The systematic review looked at 10 publications, and reported that all of the studies on CH resulted in improved skin health looking at parameters such as moisture, elasticity, wrinkle number and dryness. Studies ranged in duration from 8 weeks to 12 months, and used doses from 500 µg to 10 g/day. No analysis was made on possible dosage effects, so it is difficult to ascertain if these exist. Data quality was assessed in this study; however, it was not commented upon and so it is difficult to ascertain the overall validity of the findings.
One triple-blind RCT of 50 women supplemented with 10 g marine-derived Vinh Wellness Collagen® reported 35% reduction in wrinkles after 12 weeks, and improved skin elasticity, hydration, radiance, firmness and reduction in wrinkles compared with placebo.113 It was concluded that fish-derived CH may improve skin health in an ageing population. This assessment was based on a validated, subjective measure for skin appearance and clinic-trained staff questionnaires.
One observational study of 29 women supplemented with 1 g BioCell® collagen CH-II plus hyaluronic acid and chondroitin sulphate over 12 weeks reported a 76% decrease in skin dryness and a 13.2% decrease in wrinkles from the start of the study using visual/tactile analysis.40 Using quantifiable methods, skin collagen was shown to increase in the first 6 weeks of the study, a level that failed to be maintained to the end of the study, indicating a short-term improvement in collagen content. It was concluded that CH may affect the development of age-associated skin health.
Although the majority of the studies looked at collagen in combination or at branded collagen products, which may have implications for translatability, one double-blind RCT looked at unbranded 1 g/day CH in 64 individuals.114 CH rich in GLY-PRO-HYP and GLY-X-Y derived from fish sources reported improved skin hydration, elasticity and wrinkling compared with placebo after 12 weeks. Improvements in skin hydration were seen as early as 6 weeks, indicating a relatively short time for results. This along with a lot of the trials was based on questionnaires, which may be subject to reporting bias.
Results from the previous trial suggest that differing peptide contents may have differing actions, and one double-blind placebo-controlled RCT of 85 individuals assessed the use of CH with differing peptides derived from fish sources.115 Skin moisture, skin elasticity, the number of wrinkles and skin roughness were significantly improved in individuals taking a supplement high (2 g/kg) in PRO-HYP and HYP-GLY peptides compared with those taking a low (0.1 g/kg) peptide content and placebo after 8 weeks. In those taking a low-peptide-content supplement, improvements were only
observed in skin moisture compared with placebo. This indicates that differing peptide contents may have differing bioactivity and benefits to skin health. PRO-HYP and HYP-GLY have been reported to be precursors for collagen production.116
Many of the studies reviewed used CH from fish sources; however, one placebo-controlled parallel group study of 33 individuals over 8 weeks compared porcine CH with fish, reporting no differences in improvement of skin hydration.117 This is in contrast to other studies where the individual properties of fish-based collagen may have advantages over land-based mammals for skin health.17,18,118
One double-blind placebo-controlled RCT looked at the effects of topical CH application compared with 9 g/day oral supplementation with added vitamins A, C and E and zinc over a 90-day period.119 The oral supplement acted on skin elasticity and showed more pronounced effects on pore reduction and skin hydration than the topical application, but this was not significant. It was hypothesised in the conclusion that the two treatments may work synergistically due to differing mechanisms of action.
One placebo-controlled RCT of 2.5 g/day bioactive collagen peptides (BCPs) in the branded supplement VERISOL® reported a reduction in eye wrinkles in 114 people
compared with placebo after 8 weeks.120 There was a 65% increase in skin procollagen type I and 18% increase in elastin. Improvements to eye wrinkles were seen after 4 weeks, but were even more pronounced after 8 weeks. Moreover, these effects perpetuated 4 weeks after the trial ended with an 11.5% decrease in eye wrinkle volume in the supplement group, indicating the possible long-lasting benefits of this formula.
A second RCT of VERISOL® using 2.5 g/day and 5 g/day reported improved skin elasticity and skin moisture after 8 weeks in both treatment groups; however, measurements of skin function did not achieve statistical significance in comparison to placebo,121 indicating that although physical appearance may be improved, functional aspects of the skin were not.
Several studies were found using branded and combination formulas122–125 and, although results were promising with regards to collagen use in skin ageing, it cannot be ruled out that the results were due to synergistic effects of the ingredients in the formula. One of the combination studies reported that although all the parameters measured were significantly improved from baseline, only two of the eight parameters measured were significantly improved compared with placebo, and this was when looking at brightness and hydration.125
Specific recommendations are difficult on the use of collagen for skin health due to the number of trials using branded or combination formulas. Branded formulas do not always clarify preparation and processing methods of the collagen or other ingredients that they use, which may be problematic based on the previously reviewed bioavailability data. What is apparent is that universally these trials point to the benefits of collagen and its derivatives for the appearance of skin health and functionality whether in combination with topical products or other additives, or as monotherapy. Benefits were seen with CH doses as low as 500 µg to 10 g per day, with results apparent as soon as 6 weeks, and which may perpetuate long term. The majority of the research surrounds fish collagen, and its individual environmentally driven biocharacteristics may be responsible for the benefits observed.17,18 CH products rich in PRO-HYP and HYP-GLY may infer additional skin improvements compared with those with differing peptide characteristics. Mechanisms may include the stimulation of fibroblasts by CH bioactive peptides, free amino acids supporting the formation of collagen fibres and the production of new collagen, elastin and hyaluronic acid.124
Atopic dermatitis (AD)
Atopic dermatitis is characterised by decreased skin barrier function resulting in dryness and increased sensitisation to various allergens. Subsequent itching can induce inflammation, which perpetuates the condition. Given that the above studies demonstrated potential benefits to skin hydration and water loss, and its anti-inflammatory properties, dermatitis may benefit from collagen supplementation.
In a double-blind RCT of 17 patients with AD, 3.9 g/day CTP rich in GLY-PRO-HYP was shown to improve symptoms over 12 weeks, whereas regular CPs showed no symptom improvement throughout the study.7 Analyses were not performed between the groups; however, the results highlight that differing collagen composition of the supplement may have
varying benefits.
The literature points towards the use of 3.9 g/day collagen rich in GLY-PRO-HYP to improve dermatitis symptoms. However, additional research in larger sample sizes and in varying degrees of AD would be of benefit.
Gut health
The gut microbiome is an ecosystem comprising of bacteria, viruses and eukaryotes, which can modulate behaviour, energy homeostasis and nutrient processing.126 Gut dysbiosis occurs when this highly personalised system becomes altered, resulting in reduced diversity.126 Chronic inflammation associated with many non-communicable diseases can alter the gut microbiota and contribute to dysbiosis.127
This may exacerbate inflammation further through the overgrowth of certain species of microbiota and the production of inflammatory signalling molecules.
Many collagen products on the market are claiming improvements to gut dysbiosis and, whilst collagen may be able to modulate inflammation, the mechanisms behind gut microbiota modulation are under-researched. One study in animal models reported that administration of CPs from fish skin to high-fat diet-fed mice resulted in a decreased abundance of the inflammatory gut bacteria Erysipelatoclostridium and Alistipes.128 Lactobacillus, Akkermansia, Parabacteroides and Odoribacter species were all increased, which are associated with health benefits.129–132
Research on the use of collagen in human gut health was not evident, and so continues to be based on studies in animal models. The mechanism through which collagen could improve gut health is not evident; however, it could be hypothesised to involve modulation of inflammation and the bidirectional relationship with the gut microbiota.
Cellulite
Often seen in the thighs and buttocks, cellulite is regarded as a natural process of ageing,133 with a complex aetiology, often resulting in negative psychological implications for those who suffer from it.
In a double-blind RCT of 105 healthy females with moderate cellulite, 2.5 g/day BCPs as the brand VERISOL® was shown to improve cellulite in women of normal weight after 3 months by 5.3% and after 6 months by 9%. This was also reflected in overweight women; however, the effect was less pronounced with a 4% improvement in cellulite after 6 months.
The research in cellulite may only be translatable if using the VERISOL® branded supplement, and supports the use of long-term therapy of 2.5 g/day BCPs to improve moderate cellulite. The effects may be more pronounced and seen earlier in women of normal weight; however, overweight women may still see an improvement with longer therapy duration.
Brittle nails
Recognised as a disorder, brittle nail syndrome is caused by impaired water binding resulting in soft, dry nails that are incapable of growth.134 Although largely comprised of keratin, animal trials on collagen have demonstrated improvements to the epidermal barrier and nail moisture.135 A small amount of literature was found on the use of collagen in nail disorders.
One observational study reported that supplementation with 2.5 g/day BCPs in the form of the branded supplement VERISOL® once daily for 24 weeks resulted in a 12% increase in nail growth and a decrease in the frequency of broken nails by 42%.136 These results were further improved 4 weeks post-treatment. Assessment was conducted by a physician; however, results were observational.
Although promising, there is only a small amount of literature on the use of collagen to improve nail health, and what is available may not be translatable due to the use of a branded formulation.
Post-exercise muscle recovery and strength
Strenuous exercise can result in structural damage to skeletal muscles in the extracellular matrix, resulting in pain, swelling and reduced function.137,138 As the extracellular matrix is predominantly collagen, supplementation may provide an interesting avenue for the improvement of muscle performance and shortened recovery times following exercise.
One double-blind RCT of 24 males supplementing 20 g/day CPs reported faster exercise recovery compared with placebo over a 9-day period; however, measures of muscle soreness and indicators of muscle damage remained comparable to placebo.139 In support of this finding, a second double-blind RCT on 57 men supplementing 15 g/day CPs for 12 weeks during an exercise programme also reported no improvements to muscle pain following exercise compared with placebo; however, more pronounced improvements to strength were observed, but this remained insignificant.140 Significant body composition improvements were observed with CPs, which was attributed to passive connective tissue adaptations.
Improvements to body composition were also observed in a third double-blind RCT on 77 women supplementing 15 g/day collagen during a 12-week exercise programme, with significantly enhanced improvements to body composition compared with placebo, and improvements to hand grip strength and leg strength, although this was not significant.141
Myofibrillar muscle protein synthesis has been shown to be significantly increased in a parallel group study of 30 g/day CPs combined with exercise over 6 days,142 which could account for the improvements to body composition and muscle strength observed above. However, this study compared CPs with whey protein, which reported superior muscle protein synthesis in favour of the whey protein group.
Although a small amount of research is available, it is of high quality, and indicates that the use of 15−30 g/day CPs may enhance improvements to recovery times and body composition in those undergoing an exercise programme, but not necessarily in muscle soreness. However, results may not be as pronounced as with whey protein, although this is not definitive, as only one study made direct comparisons.
Sarcopenia
Supplementation of collagen to improve the extracellular matrix could have benefits in other conditions. Sarcopenia is an age-associated decline in muscle mass and its associated functionality,143 the onset and progression of which may be slowed by resistance exercise training,144 but this has the potential to damage what muscle remains.
One double-blind placebo-controlled RCT was found on the supplementation of collagen in sarcopenia. This study reported that 15 g/day CPs in combination with resistance exercise for 3 months resulted in lower fat mass, and increased muscle strength, bone mass and muscular control, and this effect was more pronounced than in those who were taking placebo.145 The placebo group also undertook an exercise regime. It was concluded that CP supplementation in combination with resistance exercise improved body composition in sarcopenic males. Based on previously reviewed trials, results could be due to improvements to OA and joint-related symptoms, allowing increased exercise.
Limited but promising research points to the use of 15 g/day collagen for 3 months to enhance the beneficial effects of exercise alone on muscular body composition in those with sarcopenia. However, as the research is currently only in men, the results may not be translatable into women, and further studies are warranted in other cohorts to make recommendations.
Safety
Collagen has been used for many years in the food and cosmetic industries due to its reported biological benefits, high biocompatibility and low side-effect profile.93 It has been declared by the European Food Safety Authority as safe,146 and is generally regarded as safe by the US Food and Drug Administration.147
Among the studies reviewed, there was a strong recommendation that collagen and its derivatives are safe to use in the conditions listed. No serious adverse events were recorded in the studies, and only one trial reported mild nausea, which was attributed to the treatment.113
There appear to be no contraindications for its use, other than hypersensitivity to any of the ingredients or the collagen source, and there are no known drug−nutrient interactions.148
Research is lacking on the effects of collagen supplementation during pregnancy, and so it is advised that it is not used during pregnancy and breastfeeding.
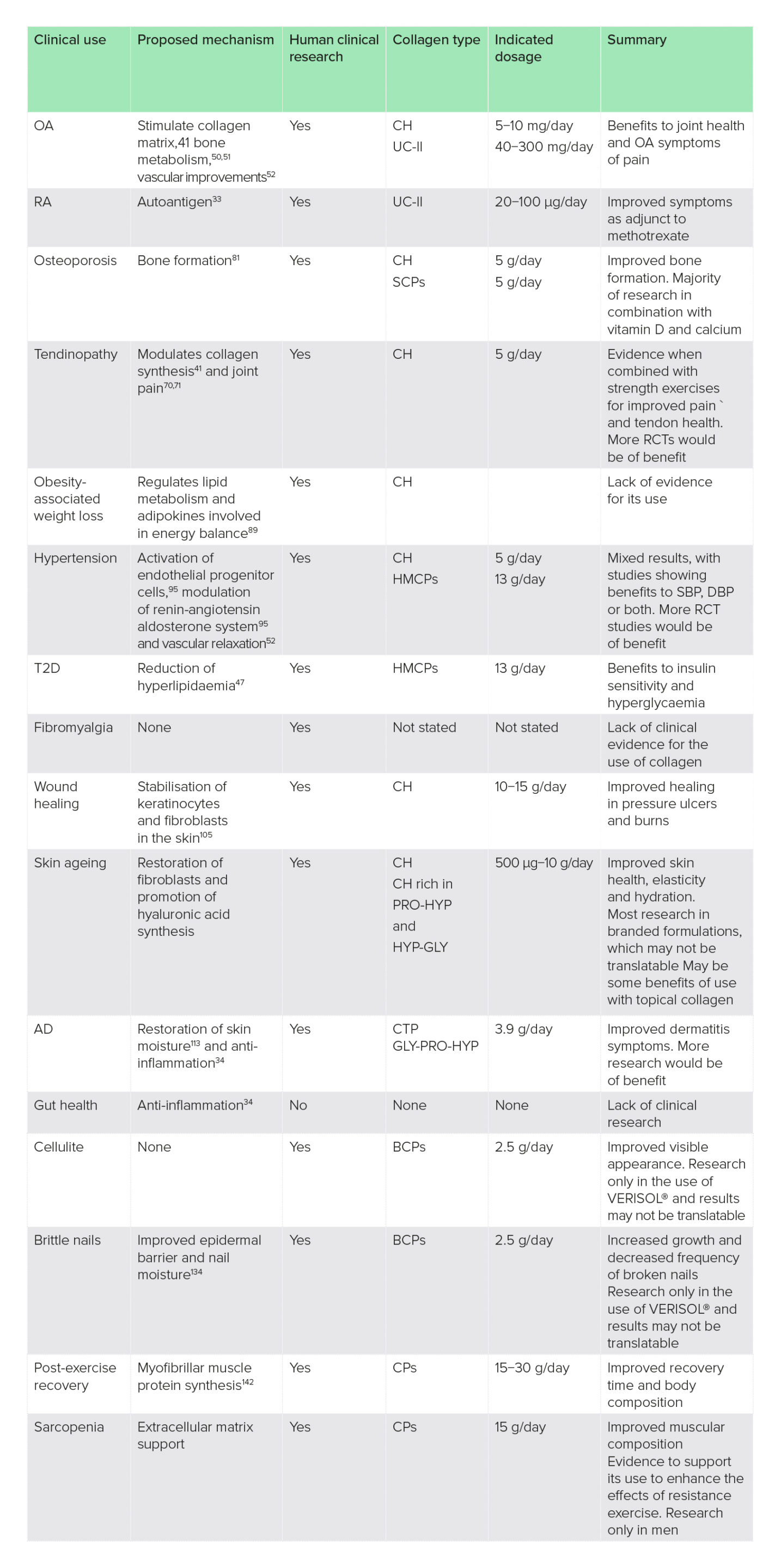
Table 1: Summary of findings on the clinical uses of collagen supplementation
AD, atopic dermatitis; BCPs, bioactive collagen peptides; CH, collagen hydrolysate; CPs, collagen peptides; CTP, collagen tripeptide; DBP, diastolic blood pressure; HMCPs, hydrolysed marine collagen peptides; OA, osteoarthritis; RA, rheumatoid arthritis; RCT, randomised-controlled trial; SBP, systolic blood pressure; SCPs, specific collagen peptides; T2D, type 2 diabetes; UC-II, unhydrolysed collagen-type II.
Conclusion
Collagen supplementation has been studied in multiple disease areas, and there is strong evidence for its therapeutic role in OA pain and function management, T2D, wound healing, skin ageing, and post-exercise body composition and strength. Mechanisms of action include structural remodelling in the skin and bones, acting as an anti-inflammatory, an antioxidant, and reducing lipotoxicity. Its high safety profile and biocompatibility make it an attractive therapy for these diseases and, although supplementary forms have been reported to have fewer amino acids than food sources, there is evidence that it is more bioavailable making it a more valuable source of collagen. Promising results have been seen for its use in osteoporosis, hypertension, sarcopenia, RA, tendinopathy, cellulite, AD and brittle nail syndrome but, due to these largely showing efficacy in combination with other therapies or due to branded forms used in the trials, it was concluded that more studies are needed to realise significant therapeutic potential. There was no evidence for the use of collagen in weight loss associated with obesity, gut health and fibromyalgia. It could be hypothesised that collagen may have a role in gut health through the modulation of inflammation; however, clinical trials were not evident and this indicates an avenue for new research (Table 1).
Difficulties arose in the study due to most of the research failing to comment on the type of collagen used or the preparation method, simply using the term hydrolysed collagen. As was previously reviewed, differing types and molecular weights of collagen and its peptides may have different bioactive properties. As a result it is recommended that healthcare professionals seek advice on the ingredients and preparation methods when recommending a product, and review the available data from human trials.
Acknowledgements
Author contributions: C. Steele carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers
and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: C. Steele has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Avila Rodríguez, M. I., Rodríguez Barroso, L. G. & Sánchez, M. L. (2018) Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol., 17(1), 20−26. doi:10.1111/jocd.12450
2 Moskowitz, R. W. (2000) Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum., 30(2), 87−99. doi:10.1053/sarh.2000.9622
3 León-López, A. et al. (2019) Hydrolyzed collagen-sources and applications. Molecules, 24(22), 4031. doi:10.3390/molecules24224031
4 Oesser, S., Adam, M., Babel, W. & Rgen Seifert, J. (1999) Nutrient Metabolism Oral Administration of 14 C Labeled Gelatin Hydrolysate Leads to an Accumulation of Radioactivity in Cartilage of Mice (C57/BL). Vol. 129, https://academic.oup.com/jn/article/129/10/1891/4721835.
5 Watanabe-Kamiyama, M. et al. (2010) Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem., 58(2), 835−841. doi:10.1021/jf9031487
6 Iwai, K. et al. (2005) Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem., 53(16), 6531−6536. doi:10.1021/jf050206p
7 Hakuta, A., Yamaguchi, Y., Okawa, T., Yamamoto, S., Sakai, Y. & Aihara, M. (2017) Anti-inflammatory effect of collagen tripeptide in atopic dermatitis. J. Dermatol. Sci., 88(3), 357−364. doi:10.1016/j.jdermsci.2017.09.002
8 Shimizu, K. et al. (2010) The bioavailable octapeptide Gly-Ala-Hyp-Gly-Leu-Hyp-Gly-Pro stimulates nitric oxide synthesis in vascular endothelial cells. J. Agric. Food Chem., 58(11), 6960−6965. doi:10.1021/jf100388w
9 Sugihara, F., Inoue, N. & Venkateswarathirukumara, S. (2018) Ingestion of bioactive collagen hydrolysates enhanced pressure ulcer healing in a randomized double-blind placebo-controlled clinical study. Sci. Rep., 8(1), 1−7. doi:10.1038/s41598-018-29831-7
10 Blanco, M., Vázquez, J. A., Pérez-Martín, R. I. & Sotelo, C. G. (2017) Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs, 15(5), 131. doi:10.3390/md15050131
11 Hossain, A., Dave, D. & Shahidi, F. (2020) Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs, 18(5), 274. doi:10.3390/md18050274
12 Sontakke, S. B., Jung, J. H., Piao, Z. & Chung, H. J. (2016) Orally available collagen tripeptide: enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J. Agric. Food Chem., 64(38), 7127−7133. doi:10.1021/acs.jafc.6b02955
13 Skov, K., Oxfeldt, M., Thøgersen, R., Hansen, M. & Bertram, H. C. (2019) Enzymatic hydrolysis of a collagen hydrolysate enhances postprandial absorption rate—a randomized controlled trial. Nutrients, 11(5), 1064. doi:10.3390/nu11051064
14 Yamamoto, S., Deguchi, K., Onuma, M., Numata, N. & Sakai, Y. (2016) Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull., 39(3), 428−434. doi:10.1248/bpb.b15-00624
15 Alcock, R. D., Shaw, G. C., Tee, N. & Burke, L. M. (2019) Plasma amino acid concentrations after the ingestion of dairy and collagen proteins, in healthy active males. Front. Nutr., 6(October), 1−11. doi:10.3389/fnut.2019.00163
16 Dallas, D. C. et al. (2017) Personalizing protein nourishment. Crit. Rev. Food Sci. Nutr., 57(15), 3313−3331. doi:10.1080/10408398.2015.1117412
17 Wang, B., Wang, Y. M., Chi, C. F., Luo, H. Y., Deng, S. G. & Ma, J. Y. (2013) Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar, Drugs, 11(11), 4641−4661. doi:10.3390/md11114641
18 Wang, L., An, X., Yang, F., Xin, Z., Zhao, L. & Hu, Q. (2008) Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem., 108(2), 616−623. doi:10.1016/j.foodchem.2007.11.017
19 Kaminaka, C. et al. (2020) Effects of low-dose Aloe sterol supplementation on skin moisture, collagen score and objective or subjective symptoms: 12-week, double-blind, randomized controlled trial. J. Dermatol., 47, 998−1006. doi:10.1111/1346-8138.15428
20 Tanaka, M. et al. (2017) Effects of aloe sterol supplementation on skin elasticity, hydration, and collagen score: a 12-week double-blind, randomized, controlled trial. Skin Pharmacol. Physiol., 29(6), 309−317. doi:10.1159/000454718
21 Tanaka, M., Misawa, E., Yamauchi, K., Abe, F. & Ishizaki, C. (2015) Effects of plant sterols derived from Aloe vera gel on human dermal fibroblasts in vitro and on skin condition in Japanese women. Clin. Cosmet. Investig. Dermatol., 8, 95−104. doi:10.2147/CCID.S75441
22 Lee, G. Y. et al. (2016) Effects of Panax ginseng extract on human dermal fibroblast proliferation and collagen synthesis. Int. Wound J., 13, 42−46. doi:10.1111/iwj.12530
23 Zhang, Y. et al. (2020) Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-ACT-mTOR pathway in osteoarthritic osteocytes. Int. J. Mol. Med., 45(4), 1225−1236.
24 Chen, X. et al. (2017) Panax ginseng total protein promotes proliferation and secretion of collagen in NIH/3t3 cells by activating extracellular signal-related kinase pathway. J. Ginseng Res., 41(3), 411−418. doi:10.1016/j.jgr.2017.02.001
25 Namgoong, S., Lee, H., Han, S. K., Lee, H. W., Jeong, S. H. & Dhong, E. S. (2019) Effect of Panax ginseng extract on the activity of diabetic fibroblasts in vitro. Int. Wound J., 16(3), 737−745. doi:10.1111/iwj.13091
26 Hashim, P. (2014) The effect of Centella asiatica, vitamins, glycolic acid and their mixtures preparations in stimulating collagen and fibronectin synthesis in cultured human skin fibroblast. Pak. J. Pharm. Sci., 27(2), 233−237.
27 Werten, M. W. T., Eggink, G., Cohen Stuart, M. A. & de Wolf, F. A. (2019) Production of protein-based polymers in Pichia pastoris. Biotechnol. Adv., 37(5), 642−666. doi:10.1016/j.biotechadv.2019.03.012
28 Myllyharju, J., Nokelainen, M., Vuorela, A. & Kivirikko, K. I. (2000) Expression of recombinant human type I-III collagen in the yeast Pichia pastoris. Biochem. Soc. Trans., 28(4), 353−357.
29 Vuorela, A., Myllyharju, J., Nissi, R., Pihlajaniemi, T. & Kivirikko, K. I. (1997) Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: Formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J., 16(22), 6702−6712. doi:10.1093/emboj/16.22.6702
30 Gellermann, P., Schneider-Barthold, C., Bolten, S. N., Overfelt, E., Scheper, T. & Pepelanova, I. (2019) Production of a recombinant non-hydroxylated gelatin mimetic in Pichia pastoris for biomedical applications. J. Funct. Biomater., 10(3), 39. doi:10.3390/jfb10030039
31 Geltor. PrimaColl. https://geltor.com/primacoll/.
32 Alcock, R. D., Shaw, G. C. & Burke, L. M. (2019) Bone broth unlikely to provide reliable concentrations of collagen precursors compared with supplemental sources of collagen used in collagen research. Int. J. Sport Nutr. Exerc. Metab., 29(3), 265−272. doi:10.1123/ijsnem.2018-0139
33 Choudhary, N., Bhatt, L. K. & Prabhavalkar, K. S. (2018) Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol., 40(3), 193−200. doi:10.1080/08923973.2018.1434793
34 Barnett, M. L. et al. (1998) Treatment of rheumatoid arthritis with oral type II collagen: Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum., 41(2), 290−297. doi:10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R
35 Tong, T. et al. (2010) Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm. Res., 59(5), 369−377. doi:10.1007/s00011-009-0109-4
36 Lerman, R. H., Chang, J.-L., Konda, V., Desai, A. & Montalto, M. B. (2015) Nutritional approach for relief of joint discomfort. Integr. Med., 14(5), 52−61.
37 Zhu, C. F., Li, G. Z., Peng, H. B., Zhang, F., Chen, Y. & Li, Y. (2010) Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab., 35(6), 797−804. doi:10.1139/H10-075
38 Barzideh, Z., Latiff, A. A., Gan, C. & Alias, A. K. (2014) ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol., 52(4), 495−504.
39 Aguirre-Cruz, G., León-López, A., Cruz-Gómez, V., Jiménez-Alvarado, R. & Aguirre-Álvarez, G. (2020) Collagen hydrolysates for skin protection: Oral administration and topical formulation. Antioxidants, 9(2), 181. doi:10.3390/antiox9020181
40 Schwartz, S. R. & Park, J. (2012) Clinical interventions in aging ingestion of BioCell Collagen®, a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin. Interv. Aging, 7, 267−273. doi:10.2147/CIA.S32836
41 Deal, C. L. & Moskowitz, R. W. (1999) Nutraceuticals as therapeutic agents in osteoarthritis. The role of glucosamine, chondroitin sulfate, and collagen hydrolysate. Rheum. Dis. Clin. North Am., 25(2), 379−395. doi:10.1016/S0889-857X(05)70074-0
42 König, D., Oesser, S., Scharla, S., Zdzieblik, D. & Gollhofer, A. (2018) Specific collagen peptides improve bone mineral density and bone markers in postmenopausal women—A randomized controlled study. Nutrients, 10(1), 97. doi:10.3390/nu10010097
43 Argyrou, C. et al. (2020) Effect of calcium and vitamin D supplementation with and without collagen peptides on bone turnover in postmenopausal women with osteopenia. J. Musculoskelet. Neuronal Interact., 20(1), 12−17.
44 Shigemura, Y. et al. (2009) Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem., 57(2), 444−449. doi:10.1021/jf802785h
45 Ohara, H. et al. (2010) Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol., 37(4), 330−338. doi:10.1111/j.1346-8138.2010.00827
46 Cicek, M. et al. (2013) Use of alpha-lipoic acid in prevention of contrast-induced nephropathy in diabetic patients. Ren. Fail., 35(5), 748−753. doi:10.3109/0886022X.2013.790298
47 Wang, J., Xie, Y., Pei, X., Yang, R., Zhang, Z. & Li, Y. (2008) The lipid-lowering and antioxidative effects of marine collagen peptides. Chinese J. Prev. Med., 42(4), 226−230.
48 Vos, T. et al. (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990−2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet, 390(10 100), 1211−1259. doi:10.1016/S0140-6736(17)32154-2
49 Bannuru, R. R. et al. (2019) OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil., 27(11), 1578−1589. doi:10.1016/j.joca.2019.06.011
50 Bailey, A. & Mansell, J. (1997) Do subchondral bone changes exacerbate or precede articular cartilage destruction in osteoarthritis of the elderly. Gerontology, 43, 296−304.
51 Nomura, Y., Oohashi, K., Watanabe, M. & Kasugai, S. (2005) Increase in bone mineral density through oral administration of shark gelatin to ovariectomized rats. Nutrition, 21(11−12), 1120−1126. doi:10.1016/j.nut.2005.03.007
52 Zhang, Y., Kouguchi, T., Shimizu, M., Ohmori, T., Takahata, Y. & Morimatsu, F. (2010) Chicken collagen hydrolysate protects rats from hypertension and cardiovascular damage. J. Med. Food, 13(2), 399−405. doi:10.1089/jmf.2009.1246
53 Saiga Egusa, A., Iwai, K., Hayakawa, T., Takahata, Y. & Morimatsu, F. (2009) Antihypertensive effects and endothelial progenitor cell activation by intake of chicken collagen hydrolysate in pre- and mild-hypertension. Biosci. Biotechnol. Biochem., 73(2), 422−424. doi:10.1271/bbb.80189
54 Faria, M., Da Costa, E. L., Gontijo, J. A. R. & Netto, F. M. (2008) Evaluation of the hypotensive potential of bovine and porcine collagen hydrolysates. J. Med. Food, 11(3), 560−567. doi:10.1089/jmf.2007.0573
55 Gunes, S., Sehim, K., Cuneyt, K., Gökmen, D. & Kuçukdeveci, A. A. (2020) Is there a relationship between venous insufficiency and knee osteoarthritis? Turkish J. Phys. Med. Rehabil., 66(1), 40−46. doi:10.5606/tftrd.2020.5110
56 Koh, S. M. et al. (2020) Elevated plasma and synovial fluid interleukin-8 and interleukin-18 may be associated with the pathogenesis of knee osteoarthritis. Knee, 27(1), 26−35. doi:10.1016/j.knee.2019.10.028
57 Saleh, K. J. & Davis, A. (2016) Measures for pain and function assessments for patients with osteoarthritis. J. Am. Acad. Orthop. Surg., 24(11), 148−162. doi:10.5435/JAAOS-D-16-00303
58 Liu, X., Machado, G. C., Eyles, J. P., Ravi, V. & Hunter, D. J. (2018) Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br. J. Sports Med., 52, 167−175. doi:10.1136/bjsports-2016-097333
59 García-Coronado, J. M. et al. (2019) Effect of collagen supplementation on osteoarthritis symptoms: a meta-analysis of randomized placebo-controlled trials. Int. Orthop., 43, 531−538. doi:10.1007/s00264-018-4211-5
60 Honvo, G., Lengelé, L., Charles, A., Reginster, J.-Y. & Bruyère, O. (2020) Role of collagen derivatives in osteoarthritis and cartilage repair: a systematic scoping review with evidence mapping. Rheumatol. Ther., 7, 703−740. doi:10.6084/m9.figshare.12987830
61 Van Vijven, J. P. J., Luijsterburg, P. A. J., Verhagen, A. P., van Osch, G. J. V. M., Kloppenburg, M. & Bierma-Zeinstra, S. M. A. (2012) Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: A systematic review. Osteoarthr. Cartil., 20(8), 809−821. doi:10.1016/j.joca.2012.04.008
62 Schauss, A. G., Stenehjem, J., Park, J., Endres, J. R. & Clewell, A. (2012) Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, biocell collagen, on improving osteoarthritis-related symptoms: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem., 60(16), 4096−4101. doi:10.1021/jf205295u
63 Benito-Ruiz, P. et al. (2009) A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr., 60(Suppl. 2), 99−113. doi:10.1080/09637480802498820
64 McAlindon, T. E. et al. (2011) Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil., 19(4), 399−405. doi:10.1016/j.joca.2011.01.001
65 Trč, T. & Bohmová, J. (2011) Efficacy and tolerance of enzymatic hydrolysed collagen (EHC) vs. glucosamine sulphate (GS) in the treatment of knee osteoarthritis (KOA). Int. Orthop., 35, 341−348. doi:10.1007/s00264-010-1010-z
66 Lugo, J. P., Saiyed, Z. M. & Lane, N. E. (2016) Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr. J., 15(14), 30−38. doi:10.1186/s12937-016-0130-8
67 Crowley, D. et al. (2009) Safety and Efficacy of Undenatured Type 2 Collagen in the Treatment of Osteoarthritis of the Knee: A Clinical Trial. http://www.medsci.org.
68 Ragle, R. L. & Sawitzke, A. D. (2012) Nutraceuticals in the management of osteoarthritis: a critical review. Drugs and Aging, 29(9), 717−731. doi:10.1007/s40266-012-0006-3
69 Bruyère, O. et al. (2012) Effect of collagen hydrolysate in articular pain: A 6-month randomized, double-blind, placebo controlled study. Complement. Ther. Med., 20(3), 124−130. doi:10.1016/j.ctim.2011.12.007
70 Clark, K. L. et al. (2008) 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin., 24(5), 1485−1496. doi:10.1185/030079908X291967
71 Czajka, A. et al. (2018) Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res., 57, 97−108. doi:10.1016/j.nutres.2018.06.001
72 Bongers, C. C. W. G. et al. (2020) Effectiveness of collagen supplementation on pain scores in healthy individuals with self-reported knee pain: A randomized controlled trial. Appl. Physiol. Nutr. Metab., 45(7), 793−800. doi:10.1139/apnm-2019-0654
73 Zhang, L. L. et al. (2008) A randomized, double-blind, multicenter, controlled clinical trial of chicken type II collagen in patients with rheumatoid arthritis. Arthritis Care Res., 59(7), 905−910. doi:10.1002/art.23824
74 Wei, W. et al. (2009) A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res. Ther., 11(6), 1−10. doi:10.1186/ar2870
75 Häuselmann, H. J. et al. (1998) Can collagen type II sustain a methotrexate-induced therapeutic effect in patients with long-standing rheumatoid arthritis? A double-blind, randomized trial. Br. J. Rheumatol., 37(10), 1110−1117. doi:10.1093/rheumatology/37.10.1110
76 Trentham, D. E. et al. (1993) Effects of oral administration of type II collagen on rheumatoid arthritis. Science, 261(5129), 1727−1730. doi:10.1126/science.8378772
77 Sieper, J. et al. (1996) Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial. Arthritis Rheum., 39(1), 41−51. doi:10.1002/art.1780390106
78 Karaguzel, G. & Holick, M. F. (2010) Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord., 11(4), 237−251. doi:10.1007/s11154-010-9154-0
79 Saito, M. & Marumo, K. (2015) Effects of collagen crosslinking on bone material properties in health and disease. Calcif. Tissue Int., 97(3), 242−261. doi:10.1007/s00223-015-9985-5
80 Viguet-Carrin, S., Garnero, P. & Delmas, P. D. (2006) The role of collagen in bone strength. Osteoporos Int., 17(3), 319−336. doi:10.1007/s00198-005-2035-9
81 Elam, M. L. et al. (2015) A calcium-collagen chelate dietary supplement attenuates bone loss in postmenopausal women with osteopenia: A randomized controlled trial. J. Med. Food, 18(3), 324−331. doi:10.1089/jmf.2014.0100
82 Bello, A. E. & Oesser, S. (2006) Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin., 22(11), 2221−2232. doi:10.1185/030079906X148373
83 Guo, L. et al. (2015) In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J. Sci. Food Agric., 95(7), 1514−1520. doi:10.1002/jsfa.6854
84 Pal, G. K. & Suresh, P. V. (2016) Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol., 37(Part B), 201−215. doi:10.1016/j.ifset.2016.03.015
85 Van Der Plas, A. et al. (2012) A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med., 46(3), 214−218. doi:10.1136/bjsports-2011-090035
86 Wright, S. M. & Aronne, L. J. (2012) Causes of obesity. Abdom. Imaging, 37, 730−732. doi:10.1007/s00261-012-9862-x
87 Kushner, R. F. (2018) Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog. Cardiovasc. Dis., 61(2), 246−252. doi:10.1016/j.pcad.2018.06.001
88 Zibellini, J. et al. (2015) Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J. Bone Miner. Res., 30(12), 2168−2178. doi:10.1002/jbmr.2564
89 Woo, M., Song, Y. O., Kang, K. H. & Noh, J. S. (2018) Anti-obesity effects of collagen peptide derived from skate (raja kenojei) skin through regulation of lipid metabolism. Mar. Drugs, 16(9), 1−12. doi:10.3390/md16090306
90 Clifton, P. M., Condo, D. & Keogh, J. B. (2014) Long term weight maintenance after advice to consume low carbohydrate, higher protein diets−a systematic review and meta analysis. Nutr. Metab. Cardiovasc. Dis., 24(3), 224−235. doi:10.1016/j.numecd.2013.11.006
91 Tak, Y. J. et al. (2019) Effect of oral ingestion of low-molecular collagen peptides derived from skate (Raja kenojei) skin on body fat in overweight adults: A randomized, double-blind, placebo-controlled trial. Mar. Drugs, 17(3), 157. doi:10.3390/md17030157
92 Giglio, B. M. et al. (2019) Whey protein supplementation compared to collagen increases blood nesfatin concentrations and decreases android fat in overweight women: A randomized double-blind study. Nutrients, 11(9), 1−14. doi:10.3390/nu11092051
93 Liu, D., Nikoo, M., Boran, G., Zhou, P. & Regenstein, J. M. (2015) Collagen and gelatin. Annu. Rev. Food Sci. Technol., 6, 527−557. doi:10.1146/annurev-food-031414-111800
94 World Health Organisation. https://www.who.int/health-topics/hypertension/#tab=tab_1.
95 Saiga-Egusa, A., Iwai, K., Hayakawa, T., Takahata, Y. & Morimatsu, F. (2009) Antihypertensive effects and endothelial progenitor cell activation by intake of chicken collagen hydrolysate in pre- and mild-hypertension. Biosci. Biotechnol. Biochem., 73(2), 422−424. doi:10.1271/bbb.80189
96 Kouguchi, T. et al. (2013) Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem., 77(4), 691−696. doi:10.1271/bbb.120718
97 Zhu, C. F., Li, G. Z., Peng, H. B., Zhang, F., Chen, Y. & Li, Y. (2010) Therapeutic effects of marine collagen peptides on Chinese patients with type 2 diabetes mellitus and primary hypertension. Am. J. Med. Sci., 340(5), 360−366. doi:10.1097/MAJ.0b013e3181edfcf2
98 Javeed, N. & Matveyenko, A. (2018) Circadian etiology of type 2 diabetes mellitus. Physiology, 33, 138−150. doi:10.1152/physiol.00003.2018
99 Organization WH. Diabetes.
100 Sears, B. & Perry, M. (2015) The role of fatty acids in insulin resistance. Lipids Health Dis., 14, 121. doi:10.1186/s12944-015-0123-1
101 Zhu, C. F., Li, G. Z., Peng, H. B., Zhang, F., Chen, Y. & Li, Y. (2010) Effect of marine collagen peptides on markers of metabolic nuclear receptors in type 2 diabetic patients with/without hypertension. Biomed. Environ. Sci., 23(2), 113−120. doi:10.1016/S0895-3988(10)60040-2
102 Wolfe, F. et al. (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum., 33(2), 160−172. doi:10.1002/art.1780330203
103 Olson, G. B., Savage, S. & Olson, J. A. (2000) The effects of collagen hydrolysate on symptoms of chronic fibromyalgia and temporomandibular joint pain. Cranio., 18(2), 135−141. doi:10.1080/08869634.2000.11746125
104 Bagheri Miyab, K. et al. (2020) The effect of a hydrolyzed collagen-based supplement on wound healing in patients with burn: A randomized double-blind pilot clinical trial. Burns, 46(1), 156−163. doi:10.1016/j.burns.2019.02.015
105 Holmes, C., Wrobel, J. S., Maceachern, M. P. & Boles, B. R. (2013) Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: A systematic review. Diabetes Metab. Syndr. Obes. Targets Ther., 6, 17−29. doi:10.2147/dmso.s36024
106 Lee, S. K., Posthauer, M. E., Dorner, B., Redovian, V. & Maloney, M. J. (2006) Pressure ulcer healing with a concentrated, fortified, collagen protein hydrolysate supplement: A randomized control trial. Wound Care, 19(92), 92−96.
107 Puizina-Ivic, N. (2008) Skin aging. Acta Dermatoven., 2, 47−54.
108 Shuster, S., Black, M. M. & McVitie, E. (1975) The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol., 93(6), 639−643. doi:10.1111/j.1365-2133.1975.tb05113.x
109 Ganceviciene, R., Liakou, A. I., Theodoridis, A., Makrantonaki, E. & Zouboulis, C. C. (2012) Skin anti-aging strategies. Dermatoendocrinology, 4(3), 308−319.
110 Sato, K. (2017) The presence of food-derived collagen peptides in human body-structure and biological activity. Food Funct., 8(12), 4325−4330. doi:10.1039/c7fo01275f
111 Cole, M. A., Quan, T., Voorhees, J. J. & Fisher, G. J. (2018) Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J. Cell. Commun. Signal., 12(1), 35−43. doi:10.1007/S12079-018-0459-1
112 Barati, M. et al. (2020) Collagen supplementation for skin health: A mechanistic systematic review. J. Cosmet. Dermatol., 19(11), 2820−2829. doi:10.1111/jocd.13435
113 Evans, M., Lewis, E. D., Zakaria, N., Pelipyagina, T. & Guthrie, N. (2021) A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol., 20(3), 825−834. doi:10.1111/jocd.13676
114 Kim, D.-U., Chung, H.-C., Choi, J., Sakai, Y. & Lee, B.-Y. (2018) Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: a randomized, double-blind, placebo-controlled study. Nutrients, 10, 826. doi:10.3390/nu10070826
115 Inoue, N., Sugihara, F. & Wang, X. (2016) Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric., 96(12), 4077−4081. doi:10.1002/jsfa.7606
116 Albaugh, V. L., Mukherjee, K. & Barbul, A. (2017) Proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr., 147(11), 2011−2017. doi:10.3945/jn.117.256404
117 Asserin, J., Lati, E., Shioya, T. & Prawitt, J. (2015) The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol., 14, 291−301.
118 Tanaka, M., Koyama, Y. I. & Nomura, Y. (2009) Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem., 73(4), 930−932. doi:10.1271/bbb.80649
119 Maia Campos, P. M. B. G., Melo, M. O. & Siqueira César, F. C. (2019) Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J. Cosmet. Dermatol., 18(6), 1693−1699. doi:10.1111/jocd.12893
120 Proksch, E., Schunck, M., Zague, V., Segger, D., Degwert, J. & Oesser, S. (2014) Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharmacol. Physiol., 27(3), 113−119. doi:10.1159/000355523
121 Proksch, E., Segger, D., Degwert, J., Schunck, M., Zague, V. & Oesser, S. (2014) Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: A double-blind, placebo-controlled study. Skin Pharmacol. Physiol., 27(1), 47−55. doi:10.1159/000351376
122 Laing, S., Bielfeldt, S., Ehrenberg, C. & Wilhelm, K. P. (2020) A dermonutrient containing special collagen peptides improves skin structure and function: a randomized, placebo-controlled, triple-blind trial using confocal laser scanning microscopy on the cosmetic effects and tolerance of a drinkable collagen supplement. J. Med. Food, 23(2), 147−152. doi:10.1089/jmf.2019.0197
123 De Luca, C., Mikhal’Chik, E. V., Suprun, M. V., Papacharalambous, M., Truhanov, A. I. & Korkina, L. G. (2016) Skin antiageing and systemic redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxid. Med. Cell Longev., 2016, 4389419. doi:10.1155/2016/4389410
124 Genovese, L., Corbo, A. & Sibilla, S. (2017) An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants. Skin Pharmacol. Physiol., 30(3), 146−158. doi:10.1159/000464470
125 Lin, P. et al. (2021) Collagen formula with Djulis for improvement of skin hydration, brightness, texture, crow’s feet, and collagen content: A double-blind, randomized, placebo-controlled trial. J. Cosmet. Dermatol., 20(1), 188−194. doi:10.1111/jocd.13500
126 Hiippala, K. et al. (2018) The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients, 10(8), 988. doi:10.3390/nu10080988
127 Ni, J., Wu, G. D., Albenberg, L. & Tomov, V. T. (2017) Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol., 14(10), 573−584. doi:10.1038/nrgastro.2017.88
128 Wang, S., Lv, Z., Zhao, W., Wang, L. & He, N. (2020) Collagen peptide from Walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J. Funct. Foods, 74(September), 104 194. doi:10.1016/j.jff.2020.104194
129 Preston, K., Krumian, R., Hattner, J., Demontigny, D., Stewart, M. & Gaddam, S. (2018) Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef. Microbes, 9(5), 697−706. doi:10.3920/BM2017.0105
130 Dao, M. C. et al. (2016) Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut, 65(3), 426−436. doi:10.1136/gutjnl-2014-308778
131 Haro, C. et al. (2016) Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J. Clin. Endocrinol. Metab., 101(1), 233−242. doi:10.1210/jc.2015-3351
132 Gomez-Arango, L. F., Barrett, H. L., McIntyre, H. D., Callaway, L. K., Morrison, M. & Dekker Nitert, M. (2016) Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension, 68(4), 974−981. doi:10.1161/HYPERTENSIONAHA.116.07910
133 Mirrashed, F., Sharp, J. C., Krause, V., Morgan, J. & Tomanek, B. (2004) Pilot study of dermal and subcutaneous fat structures by MRI in individuals who differ in gender, BMI, and cellulite grading. Skin Res. Technol., 10(3), 161−168. doi:10.1111/j.1600-0846.2004.00072.x
134 Dimitris, R. & Ralph, D. (2012) Management of simple brittle nails. Dermatol. Ther., 25(6), 569−573. doi:10.1111/j.1529-8019.2012.01518.x
135 Oba, C. et al. (2013) Collagen hydrolysate intake improves the loss of epidermal barrier function and skin elasticity induced by UVB irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed., 29(4), 204−211. doi:10.1111/phpp.12051
136 Hexsel, D., Zague, V., Schunck, M., Siega, C., Camozzato, F. O. & Oesser, S. (2017) Oral supplementation with specific bioactive collagen peptides improves nail growth and reduces symptoms of brittle nails. J. Cosmet. Dermatol., 16(4), 520−526. doi:10.1111/jocd.12393
137 Clarkson, P. & Sayers, S. (1999) Etiology of exercise-induced muscle damage. J. Appl. Physiol., 24(3), 234−248.
138 Hyldahl, R. & Hubal, M. (2014) Lengthening our perspective: morpological, cellular and molecular responses to eccentric exercise. Muscle Nerve, 49(2), 155−170. doi:10.1002/mus.21475
139 Clifford, T. et al. (2019) The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids, 49(2), 155−170. doi:10.1007/s00726-019-02706-5
140 Kirmse, M., Oertzen-Hagemann, V., de Marées, M., Bloch, W. & Platen, P. (2019) Prolonged collagen peptide supplementation and resistance exercise training affects body composition in recreationally active men. Nutrients, 11(5), 1154. doi:10.3390/nu11051154
141 Jendricke, P., Centner, C., Zdzieblik, D., Gollhofer, A. & König, D. (2019) Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: A randomized controlled trial. Nutrients, 11(4), 892. doi:10.3390/nu11040892
142 Oikawa, S. Y., Kamal, M. J., Webb, E. K., McGlory, C., Baker, S. K. & Phillips, S. M. (2020) Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: A randomized controlled trial. Am. J. Clin. Nutr., 111(3), 708−718. doi:10.1093/ajcn/nqz332
143 Malafarina, V., Úriz-Otano, F., Iniesta, R. & Gil-Guerrero, L. (2012) Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas, 71(2), 109−114. doi:10.1016/j.maturitas.2011.11.012
144 Pillard, F. et al. (2011) Physical activity and sarcopenia. Clin. Geriatr. Med., 27(3), 449−470. doi:10.1016/j.cger.2011.03.009
145 Zdzieblik, D., Oesser, S., Baumstark, M. W., Gollhofer, A. & König, D. (2015) Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr., 114(8), 1237−1245. doi:10.1017/S0007114515002810
146 EFSA (2005) Opinion of the Scientific Panel on biological hazards (BIOHAZ) on the safety of collagen and a processing method for the production of collagen. EFSA J., 174, 1−9. doi:10.2903/j.efsa.2005.174
147 FDA. Database of select committee on GRAS substances (SCGRAS) Opinion: Gelatin.
148 Therapeutics I. Drug Nutrient Interaction Database. https://www.integrativepro.com/drug-nutrient-interaction-checker.