By Karin Elgar
Abstract
Vitamin B12 (B12) is an essential cofactor in cellular metabolism − adequate supplies are needed for normal blood formation and neurological function, and deficiency can therefore lead to macrocytic anaemia and neurological deficits. Deficiency can be due to inadequate dietary intake, especially in vegans, but is otherwise more likely due to problems with absorption, which is more complex than that of other vitamins. Apart from vegans and people with gastrointestinal conditions, infants and children from B12-deficient mothers and the elderly are at particular risk of B12 deficiency.
Whilst in conventional medical practice B12 deficiency is generally treated with intramuscular injections, it is generally accepted that oral (per os) administration at a high dose is as effective at improving B12 status. Diagnosis of B12 deficiency is complicated by the fact that levels of B12 in the blood are maintained even when stores are low at the expense of tissue levels. Where a deficiency is suspected but serum levels are normal, other markers can be used to confirm a deficiency.
Benefits of B12 supplementation have been shown in a number of conditions, including diabetic neuropathy, back pain, mouth ulcers, autism spectrum disorder and elevated homocysteine levels, and it is generally considered to be very safe even at high dosages.
Cite as: Elgar, K. (2022) Vitamin B12: A review of clinical use and efficacy. Nutr Med J., 1 (3), 9-25.
Affiliation: K. Elgar is with the Nutritional Medicine Institute, London, UK.
Corresponding author: Karin Elgar (email info@karinelgar.com).
Article history: Received 27 September 2021; Peer-reviewed and received in revised form 11 January 2022; Accepted 17 March 2022. Available online 30 September 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Vitamin B12 (B12), or cobalamin, is a cobalt-containing compound, and exists in four forms, methylcobalamin (methyl-B12) and adenosylcobalamin (adenosyl-B12) that naturally occur in the body, and cyanocobalamin (cyano-B12) and hydroxocobalamin (hydroxo-B12) that are synthetic forms used in supplementation and food fortification.1 Adequate supplies are essential for normal blood formation and neurological function.2
Methyl-B12 is an essential cofactor in the one-carbon methylation cycle converting homocysteine to methionine, and in the course converting methyl-tetrahydrofolate back to tetrahydrofolate; deficiency can therefore lead to elevated homocysteine levels and impaired synthesis of DNA.3 The latter affects blood formation, leading to anaemia, a hallmark sign of B12 deficiency.3
Adenosyl-B12, on the other hand, is a cofactor for the enzyme converting methylmalonyl-CoA to succinyl-CoA, which is crucial for channelling fatty acids and amino acids into the Krebs cycle for energy production.3 In B12 deficiency, methylmalonic acid (MMA) can accumulate, and it is thought that elevated MMA, alongside elevated homocysteine, contributes to damage of the myelin sheath (which protects nerve cells and speeds up nerve transmission), which can cause irreversible neurological problems.3
Vitamin B12 deficiency
The haematological effects of B12 deficiency are the same as for folate deficiency, and are characterised by macrocytic anaemia (with abnormally large red blood cells).2 Folate supplementation can correct these haematological effects of B12 deficiency but not the neurological complications, which include neuropathies, such as tingling and numbness in the arms and legs, sensory and motor disturbances, including abnormal gait, and cognitive changes.2 Seventy-five−90% of patients with B12 deficiency present with neurological complications, which may be the only manifestation in up to 25% of cases.2
B12 deficiency can be due to inadequate dietary intake, in particular in people following a strict vegan diet (B12 is only found in animal foods). As B12 is stored in the liver, it can take 3 years following a switch to a vegan diet before deficiency occurs.
More commonly though, deficiency is due to poor absorption of this large vitamin. Stomach acid and pepsin are required to free food-bound B12, which then binds to salivary R-proteins. Pancreatic proteases release B12 in the small intestine where it then binds to intrinsic factor (IF), which is secreted by the parietal cells in the stomach. The IF−B12 complex is absorbed in the ileum, the distal part of the small intestine.2 If anything goes wrong with any of these steps, such as low stomach acid (e.g. due to ageing or through acid-lowering medication), or bariatric surgery, lack of pancreatic enzymes, small intestinal bacterial overgrowth, certain intestinal parasites or dysfunction of the small intestine (e.g. in coeliac or Crohn’s disease), this can lead to B12 deficiency.2 Metformin (an antidiabetic drug) and nitric oxide have also been shown to decrease B12.4,5,6
The term ‘pernicious anaemia’ refers to an autoimmune disease that destroys the parietal cells, leading to a lack of IF and thus B12 deficiency.7
Apart from vegans and people with gastrointestinal conditions, infants and children from B12-deficient mothers and the elderly are at particular risk of B12 deficiency, and pregnant and breastfeeding women have higher requirements.4
Diagnosis of deficiency
There is no simple definitive laboratory test to establish B12 deficiency.
After absorption, B12 is bound to transport proteins, 75−80% to haptocorrin, which transports B12 in the blood, and 20−25% to transcobalamin, which delivers B12 to the tissues, also referred to as ‘active B12’ or ‘holo-transcobalamin’.4 Both total B12, i.e. bound to haptocorrin and transcobalamin, can be tested in serum. However, serum levels may be maintained at normal or borderline levels at the expense of tissue concentrations.2
Serum levels of 300 ng/l and above are usually considered normal.3 The UK National Institute for Health and Care Excellence (NICE) states that a cut-off of 200 ng/l identifies 97% of people with B12 deficiency, and a level below 100 ng/l is usually accompanied by metabolic or clinical evidence of B12 deficiency.8 A full blood count, to check for macrocytic anaemia and other haematological parameters, should also be carried out.8
Where results are unclear, serum MMA and homocysteine levels (both of which accumulate with B12 deficiency as discussed above) can also be used to confirm a diagnosis, although homocysteine is not specific to B12 deficiency, as it may also be caused by deficiencies in other B vitamins.2,3
Correction of deficiency – different routes of administration
Depending on the cause of the deficiency, different strategies to correct it can be applied, although in conventional medical practice the most common treatment is intramuscular (i.m.) injection of B12.9 Where IF is present, a deficiency can easily be overcome by oral supplementation of crystalline cobalamin, which does not need to be cleaved from food proteins.2
Clinical trials have shown that approximately 1% of crystalline B12 is absorbed passively, i.e. without the need for IF, and that high doses (1000 µg per day) of oral B12 can therefore be effective even in pernicious anaemia.2,9,10 At the start of oral vitamin B12 replacement therapy, close monthly monitoring of laboratory results and symptoms is recommended, thereafter annual monitoring should suffice; however, patients with severe neurological complications or critically low B12 status should be treated with i.m. injections initially to rapidly replenish stores.10 Compared with i.m. B12, oral therapy could not only save resources and time, but may also be preferable for patients with coagulation issues (coagulopathies or on blood-thinning medications) and those who are adverse to injections, for example, elderly patients with sarcopenia for whom i.m. injections are painful, whilst for those with poor compliance with oral intake, injections may be preferable.10
A number of studies have investigated sublingual administration, and all found sublingual B12 to be effective in normalising B12 status in deficient adults,11,12,13,14 infants15 and children.16 Most studies included patients with various aetiologies of B12 deficiency, including pernicious anaemia, and improvements were seen across different causes. Dosing regimens have ranged from 350 µg per week (in vegetarians) to 2000 µg per day in a study including patients with pernicious anaemia, with the most common daily dose being 1000 µg per day. A number of clinical trials compared sublingual with i.m. administration and found comparable results.16,17
It is unclear whether B12 is absorbed via the oral mucosa or in the small intestine with sublingual administration, and research into this question is scarce.18 A study in 2003 compared oral versus sublingual cyano-B12 (500 µg per day for 8 weeks), and found that both increased B12 levels significantly without a significant difference between the two.13 Another study, comparing oral and sublingual administration of a complex of B12, folate and vitamin B6 also found no significant differences in effect on homocysteine between the two modes of administration.19
Correction of deficiency – different formulations of B12
As mentioned above, cobalamin exists in various forms. The most commonly used form for supplementation, both injectable and oral, is cyano-B12, which contains a cyanide moiety attached to cobalamin.7 Cyano-B12 can be converted to methyl-B12 and adenosyl-B12.2 Other commercially available forms include the naturally occurring methyl-B12, and less commonly adenosyl-B12, and hydroxo-B12, which is used mainly in prescription products.
In 2015, Obeid et al. reviewed the evidence as to whether any of the available forms of B12 are superior, and concluded that all forms can be converted to the active methyl- and adenosyl-B12 forms, and that there was not sufficient evidence to suggest that any form was superior in terms of bioavailability, biochemical effects or clinical efficacy.20
In the same year, Thakkar and Billa also compared the benefits of the different forms, and argued that methyl-B12 alone may not address the neurological complications in B12 deficiency that arise partly from the lack of adenosyl-B12.21 However, as all supplemented forms are converted to cobalamin, which then gets converted to either the methyl or adenosyl form,18 this does not appear to be plausible. The authors suggest that either methyl-B12 and adenosyl-B12 should be given together, or either cyano-B12 or hydroxo-B12 on their own, both of which can be converted into both active forms. The authors also point out that in some rare genetic disorders of B12 metabolism, the active forms may be required, and that smokers, who tend to have an excess of thiocyanate in their blood, may benefit from a taking a form other than cyano-B12.20
In 2017, Paul and Brady came to similar conclusions that all four forms appear to be effective, although they point out research that shows that tissue retention of cyano-B12 is lower and urinary excretion higher than that of the other forms, although this statement is based on a review from 1965.18 As Thakkar and Billa, they point out potential concerns for smokers, but again this is based on an article from 1970. Paul and Brady also note that various single-nucleotide polymorphisms (SNPs) may influence the efficacy and bioavailability, but that at this time there is neither sufficient clinical research in this area nor are tests for these SNPs commercially available.18
Support for the poorer bioavailability of cyano-B12 comes from a study comparing sublingual cyano- with methyl-B12 (both 1000 µg per day), which found that both forms led to a normalisation of B12 status, but B12 levels increased significantly more in the methyl-B12 form compared with the cyano-B12 form, although the formulations also had other differences − the former was a tablet, the latter a spray.16
The aim of this paper is to review the evidence for the use of B12 in clinical practice beyond the replacement in deficiency. Not all articles specify which form of B12 was used in the respective study; where the form is reported, it is stated in this paper.
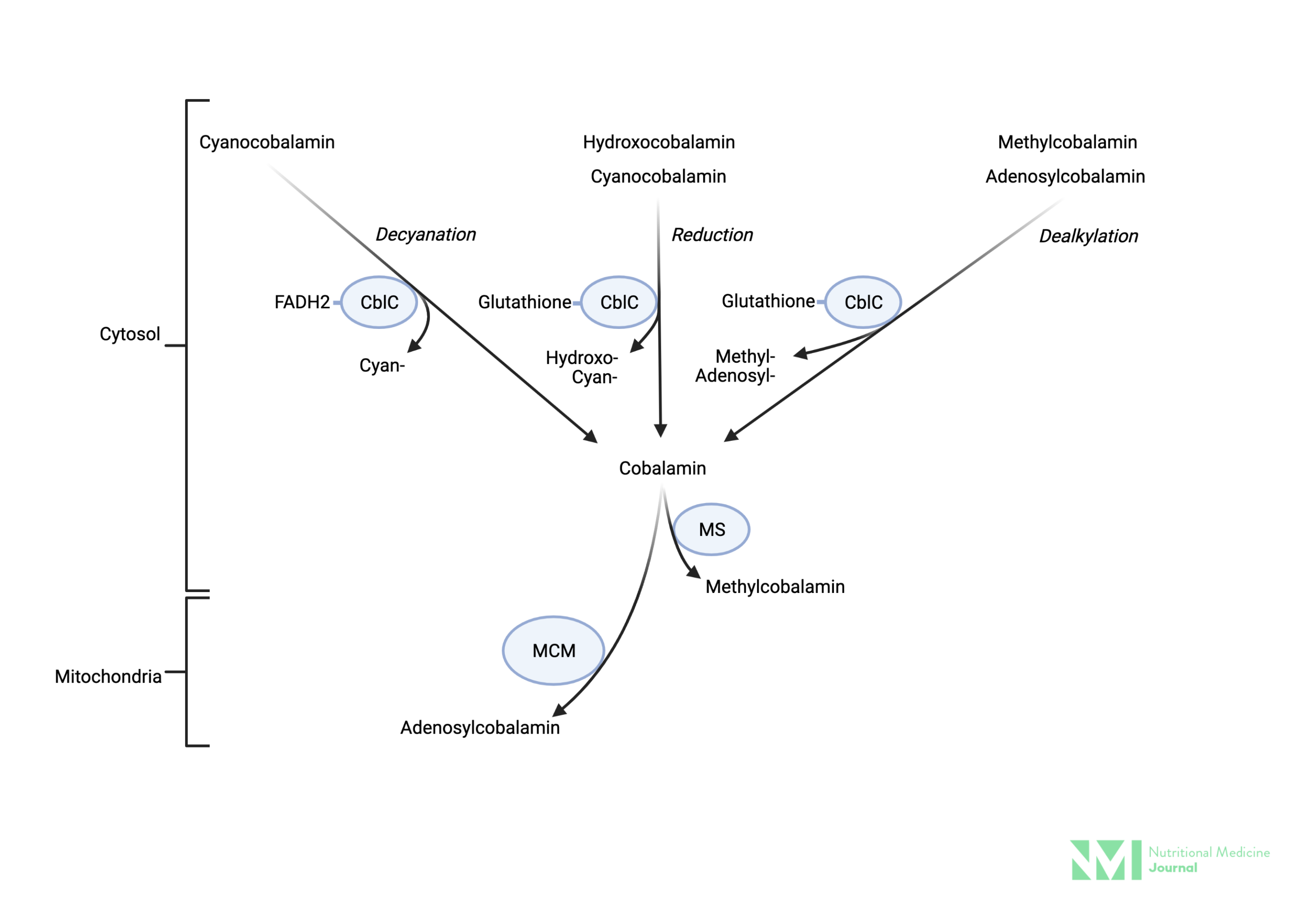
Image 1: Cellular metabolism of various forms of vitamin B12
Image: Simplified depiction of the metabolism of various forms of vitamin B12 supplements in the cytosol. Vitamin B12 is reduced to cobalamin regardless of form (cyan-, methyl-, adenosyl-, hydroxo-) then re-assembled as methylcoblamin by MS for use in the cytosol, or adenosylcobalamin by MM for use in the mitochondria. Key: CblC; cytosolic chaperon, methylmalonic aciduria, and homocystinuria type C protein FADH2; Dihydroflavine-adenine dinucleotide, MM-CoA; methylmalonyl-CoA, MS; methionine synthase, MCM: methylmalonyl-CoA mutase.
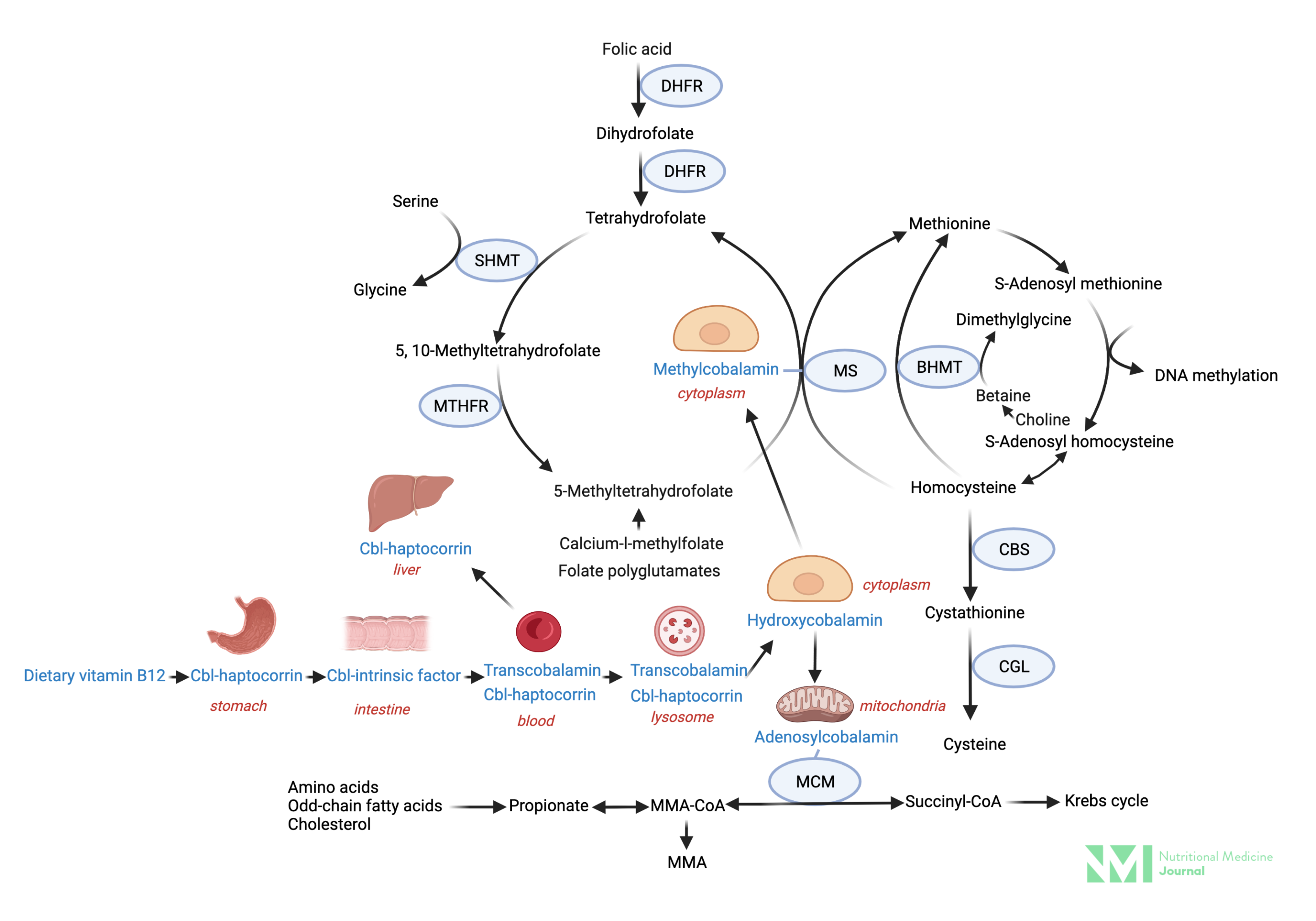
Image 2: Vitamin B12 absorption and metabolism
Image: Simplified vitamin B12 absorption and metabolism and inter-relationship with the folate cycle. Key: Cbl; cobalamin, DHFR; dihydrofolate reductase, MTHFR; methylene tetrahydrofolate reductase, SHMT; serine hydroxymethyltransferase, MS; methionine synthase, CBS; cystathionine β-synthase, CGL; cystathionine gamma lyase, NADPH; nicotinamide adenine dinucleotide phosphate, IGC-II; ntestinal glutamate carboxypeptidase II (folate hydrolase), MMA; methylmalonic acid, MM-CoA; methylmalonyl-CoA, MCM; methylmalonyl-CoA mutase.
Clinical uses
Aphthous ulcers
Aphthous (mouth) ulcers are common lesions in the oral mucosa, affecting up to 50% of the general population.22 The causes of aphthous ulcers are unknown, and may include systemic diseases, nutritional deficiencies, food allergies, genetic predisposition, immune disorders, medications and human immunodeficiency virus infection.23 As early as 1967, the benefits of i.m. B12 for healing of the oral mucosa were shown in a clinical study in patients following oral surgery.24
A study in 2008 showed that increasing B12 levels through i.m. cyano-B12 decreased the frequency of recurrent aphthous ulcers in patients with normal (defined as a B12 status of ≥ 140 ng/l) as well as with low levels of B12 at baseline.25 The dosing schedule was 1000 µg daily for 7 days, then 1000 µg once per week for 1 month, then 1 × 1000 µg injection monthly for 6 months. In 2009, a double-blind randomised-controlled trial (RCT) found significant reductions in duration of outbreaks, the number of ulcers and the level of pain in those on B12, 1000 µg per day sublingually (form not reported), regardless of B12 level.23 After 6 months of supplementation, 74% in the B12 group had reached “no aphthous ulcer status”, compared with 32% in the placebo group. In 2015, another double-blind RCT reported significant improvements in pain associated with aphthous ulcers with a B12 ointment compared with a B12-free ointment.22
Whilst evidence is limited, it consistently reports a benefit of B12 for aphthous ulcers regardless of B12 status. Both sublingual and injectable forms have shown benefits.
It has been thought that B12 deficiency may play a role in the development of aphthous ulcers.25 Volkov et al., who found that benefits were regardless of B12 status, discuss the possibility that patients in their study were deficient despite serum levels being within normal limits, as no other biomarker tests were carried out.23
Autism spectrum disorder (ASD)
Autism spectrum disorder is a common neurodevelopmental disorder, characterised by reduced social communication, and restrictive and repetitive behaviours and interests.26 Biochemical abnormalities commonly seen in patients with ASD include impaired methylation, sulphation capacities and low glutathione (GSH) redox capacity, all of which can potentially respond to B12 supplementation.26
A review of 17 clinical studies of various trial designs, including pro- and retrospective, concluded that “B12, particularly subcutaneously injected mB12 [methyl-B12], improves metabolic abnormalities in ASD along with clinical symptoms”.26 Only two of the reviewed studies were RCTs of B12 on its own in children with ASD, both using subcutaneous methyl-B12. One of the RCTs found significant mean improvements in Clinical Global Impressions-Improvement (CGI-I) score and cellular methylation capacity, but not in the Aberrant Behaviour Checklist (ABC), Social Responsiveness Scale (SRS) or GSH metabolism, with those who had the lowest methionine status at baseline (suggesting impaired methylation capacity) significantly more likely to respond to supplementation.27 The other RCT found no significant mean differences between B12 and placebo; however, it did report a significant clinical response in 30% of children on B12, which correlated with significant improvements in GSH redox status following B12 injections.28 Dosages were 64.5 and 75 µg/kg bodyweight every 3 days for 6 and 8 weeks, respectively.
Overall, although limited, the evidence suggests a benefit of methyl-B12 for children with ASD. Certain subgroups may be more likely to respond to treatment, including those with lower methionine levels and/or lower GSH redox status.
The study by Hendren et al. suggests that improvements in methylation may explain the benefits of B12 in ASD,27 whilst the results from Bertoglio et al. point towards the positive effect of B12 on GSH redox capacity as a possible mechanism of action28 that may improve brain function as well as the immune system and the gastrointestinal tract in individuals with ASD.26 Other possible mechanisms include improvements in mitochondrial function and a reduction of glutamate, an excitatory neurotransmitter, which is also a precursor to GSH.26 People with ASD have been shown to be more likely to have certain SNPs affecting B12 bioavailability, therefore they may require higher intakes.26
Cognitive function [including Alzheimer’s disease (AD)]
B12 deficiency is common in the elderly, with a prevalence of 15−40%, especially in the elderly with mental disease.29 Due to its importance in the synthesis of myelin and neurotransmitters, as well as in keeping homocysteine levels low, all of which are associated with cognitive function, B12 has been studied for a potential role in cognitive function.30
A 2021 meta-analysis on the effects of B12 on cognitive function found no benefit with either B12 alone (based on four RCTs) or B12 combined with other B vitamins (based on 13 studies).30 Three of the four RCTs of B12 alone used oral B12 (1000 µg per day) and one used i.m. B12 (1000 µg per week). In 2003, a Cochrane review of three RCTs on cognitive function in patients with AD or other forms of cognitive impairment also found no evidence of benefits of B12 on cognitive function compared with placebo.31
Since the 2021 review, one more RCT has been published that compared oral B12 alone, 25 µg per day (form not reported), or with folate (800 µg per day) versus no supplements for 6 months in patients with mild cognitive impairment.32 B12 with folate, but not alone, led to significant improvements in cognitive function.
In the 1990s and 2000s, further non-RCT studies in the elderly with or without dementia/AD and low B12 status have also mostly shown no benefit of B12 for cognitive function.33,34,35,36,37
One study suggests that length of time from diagnosis of cognitive impairment is important for response rate, in that patients who had experienced symptoms for less than 12 months improved on B12, whilst those with symptoms for more than 12 months continued to decline.38 The dose was 1000 µg i.m. daily for 1 week, once a week for 1 month, and then monthly for at least 6 months. Similarly, another study found that hydroxo-B12 (1000 µg i.m. every other day 10 times, then once a month) was of benefit for people with mild to moderate but not for those with severe dementia.29 A study in the elderly without cognitive impairment also found significant improvements with hydroxo-B12, 1000 µg i.m. weekly for 1 month, then monthly for 4 months, in cerebral and cognitive function, which were related to reductions in homocysteine.39
Most studies used B12 i.m., with slightly varying dosing regimens, commonly 1000 µg daily for 5−7 days, followed by weekly injections for another 3−4 weeks, and then monthly injections. In many of the above studies, patients were reported to have subnormal B12 levels.
Overall, there is not sufficient evidence to recommend B12 on its own for the elderly with impaired cognitive function, although those with early symptoms may benefit. This may be due to damage induced by inadequate B12 supply and affecting brain function, becoming irreversible after a certain period of time.
Depression
Major depressive disorder (MDD) affects 4.2–17% of populations worldwide, and is a common cause of disability.40 High homocysteine is associated with poor response, and high B12 levels with a good response to anti-depressant treatment.40
Four RCTs have evaluated the effects of B12 in depression. One study, which also evaluated cognitive function in patients with high MMA concentrations, found no significant effects on the MDD inventory score, but only 30 of the 140 subjects had depression at baseline, which may have diluted any benefits.41 Patients received 1000 µg cyano-B12 per week i.m. for 4 weeks or placebo injections. A study in patients with irritable bowel syndrome or inflammatory bowel disease with concomitant fatigue and depressive symptoms at baseline also found no benefits with oral B12, 1000 µg per day for 8 weeks (form not reported), on depressive symptoms despite significantly raising B12 levels, although baseline B12 levels were considered normal in all patients (> 203 ng/l).42 Another RCT studied cyano-B12, 1500 µg three times per day for 2 weeks (route not reported), in patients with seasonal affective disorder and found no improvements in either the B12 or the placebo group.43
One RCT evaluated B12 alongside anti-depressants in patients with depression and moderately low B12 status (190−300 ng/l) and found that 100% of those on B12, 1000 µg per week i.m. for 6 weeks (form not reported), responded to treatment compared with 69% on anti-depressants alone, a statistically significant difference.40
There is insufficient evidence to suggest that B12 on its own is beneficial in depression.
Fatigue
Fatigue is a common symptom of B12 deficiency, but evidence that B12 beyond the correction of deficiency has any benefits for energy is lacking.
A double-blind, placebo-controlled trial in 95 patients with irritable bowel syndrome or inflammatory bowel disease and normal B12 levels found no significant improvements in fatigue, although B12 levels increased in the B12 group who received 1000 µg per day per os (p.o.) for 8 weeks (form not reported).42 A double-blind crossover study from 1973 in patients complaining of tiredness found benefits of hydroxo-B12, 5000 µg twice per week i.m. for 2 weeks, on happiness and general wellbeing scores, but not on energy, sleep or appetite.44
An open-label, uncontrolled trial in 51 patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) found improvements in 34 patients after 3 months of supplementation with hydroxo-B12, 10 000 mcg twice per week as nasal drops. In this study, B12 levels were 244 pmol/l or more in all patients at baseline, but in those who improved, B12 levels almost quadrupled, whilst in non-responders B12 levels went up by only 50%.45 Because this study had no control group it is impossible to establish whether the observed benefits were due to a placebo effect. Nasal administration is not well studied,1 and the difference in effect on B12 levels amongst individuals may be due to the route of administration.
At this point, there is insufficient evidence to recommend B12 supplementation for fatigue beyond correcting a deficiency.
Homocysteine
Elevated homocysteine is a risk factor for cardiovascular disease, and folate, B12 and vitamin B6 have been shown to reduce homocysteine levels.46 This section will look at the effectiveness of B12 in lowering homocysteine either on its own or the additional effect it has with other B vitamins.
There is a significant body of research, mostly from the 2000s, on the homocysteine-lowering benefits of B12 in patients with end-stage renal disease and who are on haemodialysis (HD), 80−100% of whom have elevated homocysteine levels.47
Most studies in patients on HD have shown benefits of B12 either on its own or extra benefit when added to other B vitamins (mostly folate).47,48,49,50,51,52,53,54 All these studies used injectable B12, most commonly given intravenously (i.v.) after HD two−three times per week, and the most common dose was 1000 µg per injection. The forms of B12 were not always reported, but included cyano-, hydroxo- and methyl-B12.
A couple of studies found no benefits of B12 for lowering homocysteine in HD patients, one used oral B12, 2000 µg three times per week for 6 weeks,55 the other used methyl-B12 i.m. at a dose of 500 µg twice a week,56 a dose lower than in most other trials. Another RCT found a decrease in homocysteine with 1000 µg hydroxo-B12 monthly for 3 months by 2 µmol/l, whilst the control group had a 2 µmol/l increase, although the difference was not statistically significant; however, the trial was powered to only detect much larger reductions.57
Positive effects of B12 on homocysteine levels have also been observed in other patient populations, including healthy adults,46 vegetarian women,58 generally healthy women,59 older adults with elevated MMA and homocysteine,37 and patients with type 2 diabetes mellitus.60 Dosages ranged from 2 µg per day p.o. for 12 months in healthy women59 to 1000 µg per day i.m. for 3 weeks in diabetics.60 Two studies used methyl-B12,58,60 the others did not mention the form.
Overall, the evidence supports the use of B12, on its own or in addition to other supplements, for the reduction of homocysteine levels. The dosing regimen may depend on the specific patient population, with low-dose, long-term supplementation possibly being sufficient in healthy individuals, whilst patients with end-stage renal disease probably need higher dosages and ideally in combination with folate.
Pain
Animal studies have shown beneficial effects of B12 on the regeneration of nerves and the inhibition of pain-signalling pathways, including cyclooxygenase enzymes.61 Clinical trials in humans have evaluated the benefits of B12 in a number of painful conditions.
Back pain
Low back pain is very common, it is estimated that 84% of the general population experience low back pain at least once in adulthood, with up to 90% being non-specific and without known cause.62
Two double-blind RCTs investigated the effectiveness of B12 in adults who had been suffering from low back pain for more than 6 months, and both found significantly better pain reduction in the B12 compared with the placebo group. Both studies administered B12 i.m., one used methyl-B12 at a dose of 500 µg three times per week for 2 weeks,63 the other used cyano-B12, 1000 µg daily for 2 weeks.64
Positive effects of B12, 2500 µg daily sublingually for 90 days (form not reported), have also been seen in musculoskeletal pain as a side-effect of aromatase inhibitors in patients with breast cancer, with a 34% reduction of pain.65 However, as this was an open-label, uncontrolled trial, it is impossible to assess how much of the improvement was due to a placebo effect.
Although clinical trials are limited, the evidence suggests that B12 injections at a dose of at least 500 µg three times per week for 2 weeks are effective in relieving chronic low back pain.
Herpetic neuralgia
Intense neuritic pain and neuralgia are common in patients with herpes zoster (HZ, shingles), and acute herpetic neuralgia (AHN) is defined as pain within 30 days of HZ infection.66 Post-herpetic neuralgia affects one in five patients with HZ and, whilst most patients fully recover within 1 year, for some symptoms can persist for years or may be permanent.67
In 2018, a meta-analysis of four RCTs found significant benefits for herpetic neuralgia of locally injected methyl-B12, 1 mg on 6 days per week for 2−4 weeks.68 All four studies appear to have been carried out by the same group of researchers. The same research group also investigated local injection of methyl-B12 in optic herpetic neuralgia alongside local lidocaine injection versus systemic B12 administration, i.m. or p.o., alongside local lidocaine, and found the local B12 injection to be significantly more effective in pain relief than either i.m. or p.o. B12 administration.69
An RCT of 31 patients with HZ and AHN investigated the effectiveness of i.m. B12 on 3 consecutive days (dose reported as 1 mg/ml, form not reported) alongside valaciclovir (an antiviral drug) for 5 days and diclofenac (a non-steroidal anti-inflammatory drug) versus valaciclovir and diclofenac alone.70 At 3 weeks, the pain scores of both groups had significantly decreased from 5.6 and 5.1, respectively, to 0.9 in both groups, with no difference between groups. Unfortunately, pain scores were not assessed at any earlier points in this study, it is therefore unknown whether pain had decreased more in the B12 group at an earlier time point.
Whilst local injection of methyl-B12 appears to be effective in relieving herpetic pain alongside lidocaine injections, there is at present no evidence that systemic B12, either i.m. or p.o., is of benefit.
Peripheral neuropathy (PN)
In PN, nerves in the arms and legs are damaged, leading to a variety of symptoms, including pain, burning, numbness, tingling, loss of balance and co-ordination, and muscle weakness. About 1 in 10 people aged 55 years and over are affected by PN, and the most common cause is diabetes. Other causes include physical injury to nerves, viral infection such as HZ, alcohol and certain medications.71
Most research on the use of B12 focusses on diabetic neuropathy.
In the past 8 years, four meta-analyses, including 16−26 RCTs each, have pooled the data from studies comparing prostaglandin E1 (PE1) with and without B12 and with or without additional alpha-lipoic acid. The combination of B12 plus PE1 has been shown to be more effective in improving symptoms and nerve conduction velocity (NCV) than either B1272 or PE173 alone. The addition of lipoic acid further increased improvements in both clinical symptoms and NCV.74 A meta-analysis comparing B12 alone and with lipoic acid found that the combination is more effective than B12 alone.75
A number of studies on the use of B12 on its own in patients with diabetic neuropathy have been carried out, and all showed benefits in symptoms and/or neurophysiological parameters.76,77,78,79,80,81,82,83 Not all studies reported which form of B12 was used, but all that specified form had used methyl-B12. B12 was given either orally76,78,81,82 or injectable, i.m. 2000 µg twice per week for 3 months,79 i.v. 500 µg three times per week for 6 months,80 or intrathecally (into the cerebrospinal fluid) 2500 µg per month for several months.83 For oral administration, dosages were in the range of 750−1500 µg per day for 12 weeks to 1 year.
Two clinical trials investigated the benefits of B12 in non-diabetic neuropathy. An open-label RCT compared two dosing regimens, i.m. methyl-B12 500 µg three times per week versus 1500 µg once a week for 2 weeks, in 24 patients with PN and 10 healthy volunteers.84 A significantly higher increase in B12 levels was seen in both patients with PN and healthy volunteers with the three times per week compared with once a week administration. Both dosage regimens showed significant benefits in PN symptoms compared with baseline with no significant difference between the groups.
Another open-label trial involving 14 patients with immune-mediated or hereditary neuropathy evaluated i.v. methyl-B12, 25 mg daily for 10 days followed by 25 mg monthly for 5 months. Neuropathy scores improved in seven patients, mostly in patients with immune-mediated neuropathy, but remained unchanged or worsened in the remaining five patients (two patients dropped out due to adverse events that were considered unrelated to B12).85
In a post-marketing surveillance study, methyl-B12, 750 μg per day p.o. for 4 weeks, has also shown benefit alongside pregabalin (a drug commonly used for neuropathic pain), but as this was an uncontrolled trial the contribution B12 made to the improvements cannot be established.86
Overall, the evidence is overwhelmingly in favour of the use of methyl-B12, alone or alongside other treatments, in diabetic neuropathy, with oral and injectable B12 showing benefits. Evidence from RCTs for the use of B12 in other types of neuropathies is scarce, although non-randomised/uncontrolled studies have shown some benefits.
Sleep−wake cycle
In the 1990s, a number of trials were carried out into the effects of B12 on circadian rhythm and the sleep−wake cycle, but no more recent studies have been published on this topic.
A number of small studies under laboratory conditions showed that B12 can alter melatonin levels and affect circadian rhythm.87,88,89
Results from studies on whether B12 can improve sleep−wake rhythm have been mixed. Whilst some studies found beneficial effects in patients with AD36 and dementia90 and in shift workers,91 others found no improvements in patients with sleep−wake rhythm disorders.92,93 However, in the latter patient population, B12 has shown some benefits when used alongside other approaches including light therapy and drugs, although these studies had some methodological weaknesses, making it difficult to interpret the results.94,95 All of the studies had small sample sizes, and study details are mostly not well reported.
With no further research available at the moment, there is insufficient evidence to recommend B12 for sleep rhythm disorders.
The mechanism behind the possible influence of B12 on the circadian rhythm is not clear, but there is evidence to suggest that B12 may increase the light sensitivity of the circadian clock.96
Safety
B12 is considered safe even at high doses, and no adverse effects have been associated with excess B12 in healthy individuals.2,97 Studies and clinical practice of treatment of pernicious anaemia with regular high-dose injections has confirmed the safety of B12 at high dosages, and therefore neither the Institute of Medicine (IOM) in the USA nor the European Food Safety Agency (EFSA) have set an upper limit.2,97 The EFSA also states that B12 has not been found to be carcinogenic or mutagenic in in vitro and in vivo studies.
Caution is advised in patients with Leber optic atrophy, a genetic disorder caused by chronic cyanide intoxication (such as from smoking, alcohol or some plants), against the use of cyano-B12 due to its cyanide moiety, whilst hydroxo-B12 is a cyanide antagonist and can therefore be used in these patients.1,2
Allergic reactions, including anaphylactic shock, are rare and happen more often with injectable forms.1 General side-effects of hydroxo-B12 listed in the British National Formulary (BNF) are diarrhoea, dizziness, headache, hot flushes, nausea, skin reactions and urine discolouration; the frequency of these reactions is unknown.98 More side-effects are listed for injectable forms.
Drug interactions
No interactions with medications are listed in the BNF or other reports.1,2,98
Pregnancy
According to the EFSA, there is no evidence for teratogenicity or adverse effects on fertility or postnatal development with B12.97 B12 requirements are increased during pregnancy.2,97
Lactation
B12 requirements are increased during breastfeeding.2,97 Human milk naturally contains B12, and levels correlate with maternal intake and B12 status.99 Daily doses of at least 50−100 µg are needed in cases of maternal deficiency, and should improve inadequate levels in the infant without exposing the infant to excessive B12.99 B12 deficiency in infants can cause anaemia, abnormal skin and hair development, convulsions, failure to thrive, mental developmental delay and possibly abnormal movements, and direct supplementation of the infant is recommended in such cases.99
Children
There are no specific safety concerns for children.
Conclusion
Whilst severe B12 deficiency has a clear clinical picture with characteristic haematological and/or neurological features, establishing mild or borderline deficiency can be problematic as serum levels are maintained at the expense of tissue concentrations, and therefore serum levels of B12 may be in the low normal or borderline range despite a deficiency. Other markers are not commonly measured either in clinical practice or in many clinical trials, making it difficult to interpret whether positive outcomes in some studies are due to correcting a deficiency or due to other pharmacological functions of B12.
There is clear evidence for the use of B12 in diabetic neuropathy, either alone or with other treatments, and in lowering homocysteine levels, again either alone or with other nutrients. Benefits have also been shown in low back pain, autistic spectrum disorders and aphthous ulcers, although this is based on small numbers of clinical trials.
There is insufficient evidence for the use of B12, beyond correction of deficiency, in cognitive impairment, depression and fatigue. However, in view of the excellent safety profile of B12, even at high doses, supplementation, ideally as part of a more comprehensive nutritional programme, may still be of value when the clinical picture and/or other factors (e.g. vegans, elderly) are suggestive of subclinical deficiency, and with borderline or low normal serum B12 when confirming a deficiency through numerous tests is not possible.
It is generally accepted that oral high-dose B12 is as effective as i.m. administration in increasing B12 levels, even with IF deficiency, although in severe deficiency i.m. administration is recommended, at least initially, to quickly normalise levels. There is no evidence that sublingual administration has any benefits over oral intake, but it may be preferable for people with swallowing problems. Cyano-B12 is the most commonly used, and cheapest, form of B12 with no substantial evidence that other forms are more effective in improving B12 status.
B12 is generally considered safe in all life stages even at high doses and there are no known interactions with any medications, but some drugs can increase the risk of B12 deficiency, including acid-lowering drugs and metformin.
Acknowledgements
Author contributions: K. Elgar carried out the literature review and formulated the manuscript.
Additonal contributions: B. Brown from the Nutritional Medicine Institute contributed Images 1 and 2. Images created with BioRender.com
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: K. Elgar has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Al Amin, A. S. M. & Gupta, V. (2021) Vitamin B12 (cobalamin). In: StatPearls [Internet]. StatPearls Publishing.
2 Institute of Medicine (1998) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Press. doi:10.17226/6015.
3 Ankar, A. & Kumar, A. (2021) Vitamin B12 deficiency. In: StatPearls [Internet]. StatPearls Publishing.
4 Obeid, R. et al. (2019) Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front. Nutr., 6, 93.
5 Yang, W., Cai, X., Wu, H. & Ji, L. (2019) Associations between metformin use and vitamin B(12) levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J. Diabetes, 11, 729–743.
6 Infante, M., Leoni, M., Caprio, M. & Fabbri, A. (2021) Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes, 12, 916–931.
7 Vasavada, A. & Sanghavi, D. (2021) Cyanocobalamin. In: StatPearls [Internet]. StatPearls Publishing.
8 National Insitute for Health and Care Excellence (NICE) (2020) Anaemia − B12 and folate deficiency. Clinical Knowledge Summary. https://cks.nice.org.uk/topics/anaemia-b12-folate-deficiency/.
9 Wang, H. et al. (2018) Oral vitamin B(12) versus intramuscular vitamin B(12) for vitamin B(12) deficiency. Cochrane Database Syst. Rev., 3, CD004655.
10 Chan, C. Q. H., Low, L. L. & Lee, K. H. (2016) Oral vitamin B12 replacement for the treatment of pernicious anemia. Front. Med., 3, 38.
11 Bensky, M. J. et al. (2019) Comparison of sublingual vs. intramuscular administration of vitamin B12 for the treatment of patients with vitamin B12 deficiency. Drug Deliv. Transl. Res., 9, 625–630.
12 Del Bo’, C. et al. (2019) Effect of two different sublingual dosages of vitamin B(12) on cobalamin nutritional status in vegans and vegetarians with a marginal deficiency: A randomized controlled trial. Clin. Nutr., 38, 575–583.
13 Sharabi, A., Cohen, E., Sulkes, J. & Garty, M. (2003) Replacement therapy for vitamin B12 deficiency: comparison between the sublingual and oral route. Br. J. Clin. Pharmacol., 56, 635–638.
14 Delpre, G., Stark, P. & Niv, Y. (1999) Sublingual therapy for cobalamin deficiency as an alternative to oral and parenteral cobalamin supplementation. Lancet (London, England), 354, 740–741.
15 Varkal, M. A. & Karabocuoglu, M. (2021) Efficiency of the sublingual route in treating B12 deficiency in infants. Int. J. Vitam. Nutr. Res., doi:10.1024/0300-9831/a000724.
16 Tuğba-Kartal, A. & Çağla-Mutlu, Z. (2020) Comparison of sublingual and intramuscular administration of vitamin B12 for the treatment of vitamin B12 deficiency in children. Rev. Invest. Clin., 72, 380–385.
17 Parry-Strong, A., Langdana, F., Haeusler, S., Weatherall, M. & Krebs, J. (2016) Sublingual vitamin B12 compared to intramuscular injection in patients with type 2 diabetes treated with metformin: a randomised trial. N. Z. Med. J., 129, 67–75.
18 Paul, C. & Brady, D. M. (2017) Comparative bioavailability and utilization of particular forms of B(12) supplements with potential to mitigate B(12)-related genetic polymorphisms. Integr. Med. (Encinitas), 16, 42–49.
19 Yazaki, Y., Chow, G. & Mattie, M. (2006) A single-center, double-blinded, randomized controlled study to evaluate the relative efficacy of sublingual and oral vitamin B-complex administration in reducing total serum homocysteine levels. J. Altern. Complement. Med., 12, 881–885.
20 Obeid, R., Fedosov, S. N. & Nexo, E. (2015) Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol. Nutr. Food Res., 59, 1364–1372.
21 Thakkar, K. & Billa, G. (2015) Treatment of vitamin B12 deficiency-methylcobalamine? Cyancobalamine? Hydroxocobalamin?-clearing the confusion. Eur. J. Clin. Nutr., 69, 1–2.
22 Liu, H.-L. & Chiu, S.-C. (2015) The effectiveness of vitamin B12 for relieving pain in aphthous ulcers: a randomized, double-blind, placebo-controlled trial. Pain Manag. Nurs., 16, 182–187.
23 Volkov, I. et al. (2009) Effectiveness of vitamin B12 in treating recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled trial. J. Am. Board Fam. Med., 22, 9–16.
24 Brunson, E. G., Smith, J. F. & Dixon, R. (1967) Vitamin B12: an aid to oral mucous membrane healing. Oral Surg. Oral Med. Oral Pathol., 24, 102–112.
25 Gulcan, E., Toker, S., Hatipoğlu, H., Gulcan, A. & Toker, A. (2008) Cyanocobalamin may be beneficial in the treatment of recurrent aphthous ulcers even when vitamin B12 levels are normal. Am. J. Med. Sci., 336, 379–382.
26 Rossignol, D. A. & Frye, R. E. (2021) The effectiveness of cobalamin (B12) treatment for autism spectrum disorder: a systematic review and meta-analysis. J. Pers. Med., 11, 784.
27 Hendren, R. L. et al. (2016) Randomized, placebo-controlled trial of methyl B12 for children with autism. J. Child Adolesc. Psychopharmacol., 26, 774–783.
28 Bertoglio, K., Jill James, S., Deprey, L., Brule, N. & Hendren, R. L. (2010) Pilot study of the effect of methyl B12 treatment on behavioral and biomarker measures in children with autism. J. Altern. Complement. Med., 16, 555–560.
29 Nilsson, K., Warkentin, S., Hultberg, B., Fäldt, R. & Gustafson, L. (2000) Treatment of cobalamin deficiency in dementia, evaluated clinically and with cerebral blood flow measurements. Aging (Milano), 12, 199–207.
30 Markun, S. et al. (2021) Effects of vitamin B12 supplementation on cognitive function, depressive symptoms, and fatigue: a systematic review, meta-analysis, and meta-regression. Nutrients, 13, 923.
31 Malouf, R. & Areosa Sastre, A. (2003) Vitamin B12 for cognition. Cochrane Database Syst. Rev., 3, CD004326. doi:10.1002/14651858.CD004326.
32 Ma, F. et al. (2019) Effects of folic acid and vitamin B12, alone and in combination on cognitive function and inflammatory factors in the elderly with mild cognitive impairment: a single-blind experimental design. Curr. Alzheimer Res., 16, 622–632.
33 van Dyck, C. H., Lyness, J. M., Rohrbaugh, R. M. & Siegal, A. P. (2009) Cognitive and psychiatric effects of vitamin B12 replacement in dementia with low serum B12 levels: a nursing home study. Int. Psychogeriatrics, 21, 138–147.
34 Kwok, T. et al. (1998) Randomized trial of the effect of supplementation on the cognitive function of older people with subnormal cobalamin levels. Int. J. Geriatr. Psychiatry, 13, 611–616.
35 Teunisse, S., Bollen, A. E., van Gool, W. A. & Walstra, G. J. (1996) Dementia and subnormal levels of vitamin B12: effects of replacement therapy on dementia. J. Neurol., 243, 522–529.
36 Ito, T. et al. (2001) Effects of vitamin B12 on bright light on cognitive and sleep-wake rhythm in Alzheimer-type dementia. Psychiatry Clin. Neurosci., 55, 281–282.
37 Garcia, A. et al. (2004) Cobalamin reduces homocysteine in older adults on folic acid-fortified diet: a pilot, double-blind, randomized, placebo-controlled trial. J. Am. Geriatr. Soc., 52, 1410–1412.
38 Martin, D. C., Francis, J., Protetch, J. & Huff, F. J. (1992) Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J. Am. Geriatr. Soc., 40, 168–172.
39 van Asselt, D. Z. et al. (2001) Cobalamin supplementation improves cognitive and cerebral function in older, cobalamin-deficient persons. J. Gerontol. A. Biol. Sci. Med. Sci., 56, M775-9.
40 Syed, E. U., Wasay, M. & Awan, S. (2013) Vitamin B12 supplementation in treating major depressive disorder: a randomized controlled trial. Open Neurol. J., 7, 44–48.
41 Hvas, A.-M., Juul, S., Lauritzen, L., Nexø, E. & Ellegaard, J. (2004) No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. J. Affect. Disord., 81, 269–273.
42 Scholten, A.-M. et al. (2018) Surplus vitamin B(12) use does not reduce fatigue in patients with irritable bowel syndrome or inflammatory bowel disease: A randomized double-blind placebo-controlled trial. Clin. Nutr. ESPEN, 23, 48–53.
43 Oren, D. A. et al. (1994) A controlled trial of cyanocobalamin (vitamin B12) in the treatment of winter seasonal affective disorder. J. Affect. Disord., 32, 197–200.
44 Ellis, F. R. & Nasser, S. (1973) A pilot study of vitamin B12 in the treatment of tiredness. Br. J. Nutr., 30, 277–283.
45 van Campen, C. L. M., Riepma, K. & Visser, F. C. (2019) Open trial of vitamin B12 nasal drops in adults with myalgic encephalomyelitis/chronic fatigue syndrome: comparison of responders and non-responders. Front. Pharmacol., 10, 1102.
46 Huang, T., Li, K., Asimi, S., Chen, Q. & Li, D. (2015) Effect of vitamin B-12 and n-3 polyunsaturated fatty acids on plasma homocysteine, ferritin, C-reaction protein, and other cardiovascular risk factors: a randomized controlled trial. Asia Pac. J. Clin. Nutr., 24, 403–411.
47 Tayebi, A. et al. (2016) Effect of Vitamin B12 supplementation on serum homocysteine in patients undergoing hemodialysis: A randomized controlled trial. Saudi J. Kidney Dis. Transpl., 27, 256–262.
48 Chiu, Y.-W., Chang, J.-M., Hwang, S.-J., Tsai, J.-C. & Chen, H.-C. (2009) Pharmacological dose of vitamin B12 is as effective as low-dose folinic acid in correcting hyperhomocysteinemia of hemodialysis patients. Ren. Fail., 31, 278–283.
49 Hoffer, L. J., Saboohi, F., Golden, M. & Barré, P. E. (2005) Cobalamin dose regimen for maximum homocysteine reduction in end-stage renal disease. Metabolism, 54, 835–840.
50 Vrentzos, G. E. et al. (2003) Intravenous administration of vitamin B12 in the treatment of hyperhomocysteinemia associated with end-stage renal disease. J. Nephrol., 16, 535–539.
51 Elian, K. M. & Hoffer, L. J. (2002) Hydroxocobalamin reduces hyperhomocysteinemia in end-stage renal disease. Metabolism, 51, 881–886.
52 Koyama, K., Usami, T., Takeuchi, O., Morozumi, K. & Kimura, G. (2002) Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high-dose folic acid supplementation. Nephrol. Dial. Transplant, 17, 916–922.
53 Kaplan, L. N., Mamer, O. A. & Hoffer, L. J. (2001) Parenteral vitamin B12 reduces hyperhomocysteinemia in end-stage renal disease. Clin. Invest. Med., 24, 5–11.
54 Dierkes, J. et al. (1999) Supplementation with vitamin B12 decreases homocysteine and methylmalonic acid but also serum folate in patients with end-stage renal disease. Metabolism, 48, 631–635.
55 Arnadottir, M. & Hultberg, B. (2003) B12. Clin. Nephrol., 59, 186–189.
56 Trimarchi, H., Schiel, A., Freixas, E. & Díaz, M. (2002) Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron, 91, 58–63.
57 Polkinghorne, K. R. et al. (2003) Randomized, placebo-controlled trial of intramuscular vitamin B12 for the treatment of hyperhomocysteinaemia in dialysis patients. Intern. Med. J., 33, 489–494.
58 Yajnik, C. S. et al. (2007) Oral vitamin B12 supplementation reduces plasma total homocysteine concentration in women in India. Asia Pac. J. Clin. Nutr., 16, 103–109.
59 Deshmukh, U. S. et al. (2010) Effect of physiological doses of oral vitamin B12 on plasma homocysteine: a randomized, placebo-controlled, double-blind trial in India. Eur. J. Clin. Nutr., 64, 495–502.
60 Araki, A., Sako, Y. & Ito, H. (1993) Plasma homocysteine concentrations in Japanese patients with non-insulin-dependent diabetes mellitus: effect of parenteral methylcobalamin treatment. Atherosclerosis, 103, 149–157.
61 Buesing, S., Costa, M., Schilling, J. M. & Moeller-Bertram, T. (2019) Vitamin B12 as a treatment for pain. Pain Physician, 22, E45–E52.
62 Mibielli, M. A. N. et al. (2020) Nucleotides cytidine and uridine associated with vitamin B12 vs B-complex vitamins in the treatment of low back pain: the NUBES study. J. Pain Res., 13, 2531–2541.
63 Chiu, C. K., Low, T. H., Tey, Y. S., Singh, V. A. & Shong, H. K. (2011) The efficacy and safety of intramuscular injections of methylcobalamin in patients with chronic nonspecific low back pain: a randomised controlled trial. Singapore Med. J., 52, 868–873.
64 Mauro, G. L., Martorana, U., Cataldo, P., Brancato, G. & Letizia, G. (2000) Vitamin B12 in low back pain: a randomised, double-blind, placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci., 4, 53–58.
65 Campbell, A., Heydarian, R., Ochoa, C., Dwivedi, A. K. & Nahleh, Z. A. (2018) Single arm phase II study of oral vitamin B12 for the treatment of musculoskeletal symptoms associated with aromatase inhibitors in women with early stage breast cancer. Breast J., 24, 260–268.
66 Xǔ, G. et al. (2016) Local injection of methylcobalamin combined with lidocaine for acute herpetic neuralgia. Pain Med., 17, 572–581.
67 NHS (2021) Post-herpetic neuralgia. Health Topics A-Z. https://www.nhs.uk/conditions/post-herpetic-neuralgia/.
68 Wang, J. Y., Wu, Y. H., Liu, S. J., Lin, Y. S. & Lu, P. H. (2018) Vitamin B12 for herpetic neuralgia: A meta-analysis of randomised controlled trials. Complement. Ther. Med., 41, 277–282.
69 Xu, G. et al. (2020) Local administration of methylcobalamin for subacute ophthalmic herpetic neuralgia: a randomized, phase III clinical trial. Pain Pract., 20, 838–849.
70 Chen, Y.-C., Lu, P.-H. & Lu, P.-H. (2021) The efficacy of intramuscular injection of vitamin B12 in the treatment of acute herpetic neuralgia−a prospective pilot study. J. Altern. Complement. Med., 27, 525–526.
71 NHS (2019) Peripheral neuropathy. Health Topics A-Z. https://www.nhs.uk/conditions/peripheral-neuropathy/.
72 Deng, H. et al. (2014) Meta-analysis of methylcobalamin alone and in combination with prostaglandin E1 in the treatment of diabetic peripheral neuropathy. Endocrine, 46, 445–454.
73 Jiang, D.-Q. et al. (2018) Prostaglandin E1 plus methylcobalamin combination therapy versus prostaglandin E1 monotherapy for patients with diabetic peripheral neuropathy: A meta-analysis of randomized controlled trials. Medicine (Baltimore), 97, e13020.
74 Jiang, D.-Q., Li, M.-X., Wang, Y. & Wang, Y. (2015) Effects of prostaglandin E1 plus methylcobalamin alone and in combination with lipoic acid on nerve conduction velocity in patients with diabetic peripheral neuropathy: A meta-analysis. Neurosci. Lett., 594, 23–29.
75 Xu, Q. et al. (2013) Meta-analysis of methylcobalamin alone and in combination with lipoic acid in patients with diabetic peripheral neuropathy. Diabetes Res. Clin. Pract., 101, 99–105.
76 Didangelos, T. et al. (2021) Vitamin B12 supplementation in diabetic neuropathy: a 1-year, randomized, double-blind, placebo-controlled trial. Nutrients, 13, 395.
77 Han, Y. et al. (2018) Differential efficacy of methylcobalamin and alpha-lipoic acid treatment on symptoms of diabetic peripheral neuropathy. Minerva Endocrinol., 43, 11–18.
78 Li, S. et al. (2016) Effects of acetyl-L-carnitine and methylcobalamin for diabetic peripheral neuropathy: A multicenter, randomized, double-blind, controlled trial. J. Diabetes Investig., 7, 777–785.
79 Talaei, A., Siavash, M., Majidi, H. & Chehrei, A. (2009) Vitamin B12 may be more effective than nortriptyline in improving painful diabetic neuropathy. Int. J. Food Sci. Nutr., 60 (Suppl 5), 71–76.
80 Kuwabara, S. et al. (1999) Intravenous methylcobalamin treatment for uremic and diabetic neuropathy in chronic hemodialysis patients. Intern. Med., 38, 472–475.
81 Yoshioka, K. & Tanaka, K. (1995) Effect of methylcobalamin on diabetic autonomic neuropathy as assessed by power spectral analysis of heart rate variations. Horm. Metab. Res., 27, 43–44.
82 Yaqub, B. A., Siddique, A. & Sulimani, R. (1992) Effects of methylcobalamin on diabetic neuropathy. Clin. Neurol. Neurosurg., 94, 105–111.
83 Ide, H. et al. (1987) Clinical usefulness of intrathecal injection of methylcobalamin in patients with diabetic neuropathy. Clin. Ther., 9, 183–192.
84 Sil, A. et al. (2018) A randomized, open labeled study comparing the serum levels of cobalamin after three doses of 500 mcg vs. a single dose methylcobalamin of 1500 mcg in patients with peripheral neuropathy. Korean J. Pain, 31, 183–190.
85 Shibuya, K. et al. (2014) Safety and efficacy of intravenous ultra-high dose methylcobalamin treatment for peripheral neuropathy: a phase I/II open label clinical trial. Intern. Med., 53, 1927–1931.
86 Dongre, Y. U. & Swami, O. C. (2013) Sustained-release pregabalin with methylcobalamin in neuropathic pain: an Indian real-life experience. Int. J. Gen. Med., 6, 413–417.
87 Mayer, G., Kröger, M. & Meier-Ewert, K. (1996) Effects of vitamin B12 on performance and circadian rhythm in normal subjects. Neuropsychopharmacology, 15, 456–464.
88 Hashimoto, S. et al. (1996) Vitamin B12 enhances the phase-response of circadian melatonin rhythm to a single bright light exposure in humans. Neurosci. Lett., 220, 129–132.
89 Uchiyama, M., Mayer, G., Okawa, M. & Meier-Ewert, K. (1995) Effects of vitamin B12 on human circadian body temperature rhythm. Neurosci. Lett., 192, 1–4.
90 Mishima, K., Okawa, M. & Hishikawa, Y. (1992) Effect of methylcobalamin (VB12) injection on sleep-wake rhythm in demented patients. Jpn. J. Psychiatry Neurol., 46, 227–228.
91 Bohr, K. C. (1996) [Effect of vitamin B12 on sleep quality and performance of shift workers]. Wien. Med. Wochenschr., 146, 289–291.
92 Takahashi, K. et al. (1999) Double-blind test on the efficacy of methylcobalamin on sleep-wake rhythm disorders. Psychiatry Clin. Neurosci., 53, 211–213.
93 Okawa, M. et al. (1997) Vitamin B12 treatment for delayed sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin. Neurosci., 51, 275–279.
94 Maeda, K. et al. (1992) A multicenter study of the effects of vitamin B12 on sleep-waking rhythm disorders: in Shizuoka Prefecture. Jpn. J. Psychiatry Neurol., 46, 229–230.
95 Yamadera, H., Takahashi, K. & Okawa, M. (1996) A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin. Neurosci., 50, 203–209.
96 Honma, K., Kohsaka, M., Fukuda, N., Morita, N. & Honma, S. (1992) Effects of vitamin B12 on plasma melatonin rhythm in humans: increased light sensitivity phase-advances the circadian clock? Experientia, 48, 716–720.
97 European Food Safety Authority (EFSA) (2015) Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA J., 13, 4150.
98 National Insitute for Health and Care Excellence (NICE) Hydroxocobalamin. British National Formulary (BNF). https://bnf.nice.org.uk/drug/hydroxocobalamin.html#indicationsAndDoses.
99 (2006) Vitamin B12. In: Drugs and Lactation Database (LactMed) [Internet]. National Library of Medicine, USA.